Treatment rechallenge is safe and leads to better survival in pancreatic cancer patients with interstitial pneumonitis
- PMID: 40108538
- PMCID: PMC11924909
- DOI: 10.1186/s12885-025-13896-5
Treatment rechallenge is safe and leads to better survival in pancreatic cancer patients with interstitial pneumonitis
Abstract
Background and aims: Interstitial pneumonitis is a potentially fatal complication of cancer-related therapy. However, data regarding the risk factors, prognosis and safety and benefit of rechallenge treatment are scarce.
Methods: Patients diagnosed with pancreatic cancer were retrospectively enrolled, and those with pneumonitis were identified. We investigated the incidence and etiology of pneumonitis, potential risk factors, and impact of treatment rechallenge on clinical outcomes.
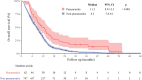
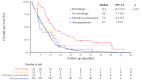
Results: A total of 809 patients were diagnosed with pancreatic cancer, among whom 62 (7.7%) were diagnosed with interstitial pneumonitis. Risk factors identified through competing risk analysis included nab-paclitaxel, gemcitabine, erlotinib, and previous lung diseases such as pre-existing ILD, asthma, chronic obstructive pulmonary disease, tuberculosis, primary lung cancer, metastasis, or pneumonia. Among these patients, 33 experienced acute respiratory distress syndrome, resulting in 15 deaths during pneumonitis episodes. After rechallenge therapy in 33 patients, pneumonitis recurred in 3 (9%). The median overall survival was longer in patients with pneumonitis than in those without. Subgroup analysis further revealed that overall survival was significantly better in the rechallenge group.
Conclusions: Most cases of pneumonitis are not directly induced by cancer therapy. Therefore, treatment rechallenge is considered a reasonable approach, potentially resulting in improved survival outcomes.
Keywords: Erlotinib; Gemcitabine; Interstitial pneumonitis; Nab-paclitaxel; Pancreatic cancer; Rechallenge.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the Declarations of Helsinki and Istanbul and approved by the Institutional Review Board (IRB) of Taipei Veterans General Hospital (approval number 2019-09-001AC). The need for consent to participate was waived by the IRB of Taipei Veterans General Hospital. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Five Cases of Interstitial Pneumonitis Due to Gemcitabine and Nab-Paclitaxel Combination Treatment in Pancreatic Cancer Patients.Pancreas. 2018 Aug;47(7):e42-e43. doi: 10.1097/MPA.0000000000001088. Pancreas. 2018. PMID: 29985849 No abstract available.
-
Interstitial lung disease in advanced pancreatic ductal adenocarcinoma patients treated with gemcitabine and nab-paclitaxel combination therapy: a retrospective analysis.Cancer Chemother Pharmacol. 2020 Mar;85(3):517-523. doi: 10.1007/s00280-019-03983-3. Epub 2019 Nov 5. Cancer Chemother Pharmacol. 2020. PMID: 31691078
-
Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study.Lancet Gastroenterol Hepatol. 2020 Mar;5(3):285-294. doi: 10.1016/S2468-1253(19)30327-9. Epub 2020 Jan 14. Lancet Gastroenterol Hepatol. 2020. PMID: 31953079 Clinical Trial.
-
Chemotherapy-induced infiltrative pneumonitis cases in breast cancer patients.J Oncol Pharm Pract. 2012 Jun;18(2):311-5. doi: 10.1177/1078155211429384. Epub 2012 Jan 4. J Oncol Pharm Pract. 2012. PMID: 22217649 Review.
-
Meta-analysis and indirect treatment comparison of modified FOLFIRINOX and gemcitabine plus nab-paclitaxel as first-line chemotherapy in advanced pancreatic cancer.BMC Cancer. 2021 Jul 23;21(1):853. doi: 10.1186/s12885-021-08605-x. BMC Cancer. 2021. PMID: 34301232 Free PMC article.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. Cancer J Clin. 2022;72(1):7–33. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. - PubMed
-
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal Irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–57. - PubMed
MeSH terms
Substances
Grants and funding
- V110C-087/Taipei Veterans General Hospital
- MOST 109-2314-B-075-030/Ministry of Science and Technology, Taiwan
- VGHUST113-G6-2-2/VGHUST Joint Research Program
- No. 113-V-B-062/Yin Shu-Tien Foundation Taipei Veterans General Hospital-National Yang Ming Chiao Tung University Excellent Physician Scientists Cultivation Program
LinkOut - more resources
Full Text Sources
Medical

