Evaluation and validation of a clinical decision support system for dose optimisation in hospitalized patients with (morbid) obesity - a retrospective, observational study
- PMID: 40108614
- PMCID: PMC11921672
- DOI: 10.1186/s12911-025-02963-3
Evaluation and validation of a clinical decision support system for dose optimisation in hospitalized patients with (morbid) obesity - a retrospective, observational study
Abstract
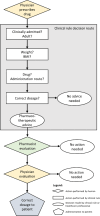
Background: Obesity changes a patient's pharmacokinetics and pharmacotherapeutic advices should be personalized to ensure proper treatment. Currently, implementations of advices regarding the obese population are lacking and weight and body mass index (BMI) are rarely monitored. The Maasstad Hospital built a clinical decision support system (CDSS) for pharmacists, based on current Dutch guidelines, to supply therapeutic advices for (morbidly) obese patients based on patient characteristics. In this study we evaluated whether patients receiving inadequate pharmacotherapy are indeed identified via this CDSS and to which extent irrelevant alerts are generated. Moreover, it is investigated to which extent pharmacists carry on the generated advices and to which extent physicians act upon these.
Methods: The research concerned a retrospective observational study performed at the Maasstad Hospital in Rotterdam, the Netherlands between January 2021 and august 2021. The drugs included were dalteparin, apixaban, dabigatran, edoxaban, rivaroxaban, vancomycin and ciprofloxacin. Dispensing data, patient characteristics and CDSS processing were collected. Dispensing data was included when the patient's weight or BMI could potentially lead to dose adjustments via the CDSS. The CDSS was evaluated for sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Additionally, excess alerts, defined as irrelevant alerts on the moment of assessment, of the CDSS and adherence to the CDSS were investigated.
Results: 1218 alerts over 3735 drug dispenses were generated. 568 alerts (46.6%) resulted in a pharmacotherapeutic advice by the pharmacist to the physician. In most cases, the sensitivity, specificity, PPV and NPV were 100.0% with varying 95% CIs. For some drugs technical adjustments were needed, including the initially incorrect BMI setting of vancomycin within the CDSS, resulting in a high excess alerts of 56.9%. Dabigatran had a NPV of 22.2% 95% CI [6.3-54.7] and a sensitivity of 56.3% 95% CI [33.2-76.9]. Overall excess alerts varied from 22.2% to 56.9%. Depending on the drug, the advices resulted in 6.9-100.0% real pharmacotherapy adjustments in practise.
Conclusion: The (morbid) obesity CDSS functions as expected and identifies the (morbidly) obese patients with inadequate pharmacotherapy. The adherence of physicians and the follow-up in practise varies widely and requires further investigation.
Trial registration: Non-WMO research W21.218.
Keywords: (Morbid) obesity; Antibiotics; Anticoagulants; Clinical decision support system; Evaluation; Hospital pharmacy; Personalized medicine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was reviewed by the Medical Research Ethics Committees United (MEC-U) September 20th 2021 and was deemed as not subject to the WMO (Medical Research Involving Human Subjects Act). The research was thereafter accepted by the local ethics committee of the Maasstad Hospital. Informed consent was waved based on article 458 of the Dutch Medical Treatment Contracts Act (WGBO). Patients were excluded if they had a registered objection against participating in scientific research in the EHR. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- World Obesity Day. 2022 – Accelerating action to stop obesity. [cited 2023 Nov 16]. Available from: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelera...
-
- Scientific American. World’s Obese Population Hits 641 Million - Scientific American. 2016 [cited 2022 Feb 9]. Available from: https://www.scientificamerican.com/article/world-s-obese-population-hits...
-
- Cleveland Clinic, Class III. Obesity (Morbid Obesity): Causes, Symptoms, Risks & Treatment. 2021 [cited 2022 Feb 9]. Available from: https://my.clevelandclinic.org/health/diseases/21989-class-iii-obesity-f...
-
- Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71–87 [cited 2021 Jun 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/20067334/ - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

