Cost-effectiveness of sacituzumab govitecan for hormone receptor-positive human epidermal growth factor receptor 2-negative metastatic breast cancer based on the EVER-132-002 trial in China
- PMID: 40108623
- PMCID: PMC11924825
- DOI: 10.1186/s12962-025-00613-z
Cost-effectiveness of sacituzumab govitecan for hormone receptor-positive human epidermal growth factor receptor 2-negative metastatic breast cancer based on the EVER-132-002 trial in China
Abstract
Background: The EVER-132-002 trial demonstrated the significant efficacy and manageable safety of sacituzumab govitecan in hormone receptor-positive human epidermal growth factor receptor 2-negative (HR + HER2-) metastatic breast cancer. This study evaluated the cost-effectiveness of sacituzumab govitecan compared with chemotherapy from the Chinese healthcare system perspective.
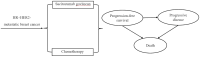
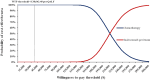
Methods: A partitioned survival model at 21-day intervals over a 10-year time horizon was developed to evaluate the total cost, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER) at willingness-to-pay (WTP) threshold of 3 times gross domestic product per capita ($38,042.49 per QALY). Clinical data were extracted from the EVER-132-002 trial; direct medical costs and utility values were obtained from public bid-winning databases, local charges or published literature. To determine the model's robustness, scenario, one-way, two-way and probabilistic sensitivity analyses were performed.
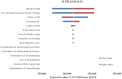
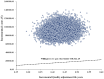
Results: Compared with chemotherapy, sacituzumab govitecan generated an additional cost of $91,273.72, with an additional QALY of 0.43, resulted in an ICER of $211,948.62 per QALY. Patient weight was the most influential parameter on base-case results, and variations in each parameter did not substantially alter the conclusion. Probabilistic sensitivity analysis demonstrated that the probability of sacituzumab govitecan to be cost-effective was zero at the WTP threshold of $38,042.49 per QALY. Scenario analysis indicated that sacituzumab govitecan would be cost-effective versus chemotherapy only if its cost was reduced by 83% ($202.65 per unit) or more.
Conclusions: Sacituzumab govitecan might not be cost-effective compared with chemotherapy in the treatment for HR + HER2- metastatic breast cancer in China.
Keywords: Cost-effectiveness; Hormone receptor-positive; Human epidermal growth factor receptor 2-negative; Metastatic breast cancer; Sacituzumab Govitecan.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

