Interactions between menopause and high-fat diet on cognition and pathology in a mouse model of Alzheimer's disease
- PMID: 40108996
- PMCID: PMC11923387
- DOI: 10.1002/alz.70026
Interactions between menopause and high-fat diet on cognition and pathology in a mouse model of Alzheimer's disease
Abstract
Introduction: Post-menopausal women constitute about two-thirds of those with Alzheimer's disease (AD). Menopause increases dementia risk by heightening the likelihood of metabolic disease, a well-known risk factor for dementia. We aimed to determine the effects of menopause and high-fat diet (HF) on cognitive and pathological outcomes in an AD mouse model.
Methods: At 3 months old, AppNL-F mice received 4-vinylcyclohexene diepoxide (menopause model) or vehicle and were placed on a control (10% fat) or an HF diet (60% fat) until 10 months old.
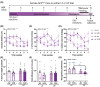
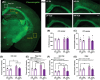
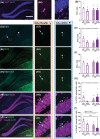
Results: An interaction between HF diet and menopause led to impaired recognition memory. No effects of menopause were observed on amyloid pathology. However, menopause induced alterations in microglial response, white matter, and hippocampal neurogenesis.
Discussion: This work highlights the need to model endocrine aging in animal models of dementia and contributes to further understanding of the interaction between menopause and metabolic health in the context of AD.
Highlights: The combination of menopause and HF diet led to early onset of cognitive impairment. HF diet increased amyloid pathology in the hippocampus. Menopause led to an increase in microglia density and a decrease in myelin in the corpus callosum. Menopause altered hippocampal neurogenesis in a diet-dependent manner.
Keywords: Alzheimer's disease; amyloid; menopause; metabolic disease; microglia; neurogenesis; white matter.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors have no conflicts to report. Author disclosures are available in the Supporting information.
Figures


References
-
- Livingston G, Costafreda SG. Interventions to prevent dementia should target those at high risk. Lancet. 2023;402(10404):750‐751. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

