Functionalized Cerium Oxide Nanoparticles Enhance Penetration into Melanoma Spheroids In Vivo through Angiogenesis
- PMID: 40109098
- PMCID: PMC12057615
- DOI: 10.1002/adhm.202405129
Functionalized Cerium Oxide Nanoparticles Enhance Penetration into Melanoma Spheroids In Vivo through Angiogenesis
Abstract
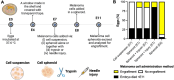
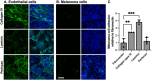
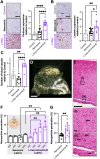
Angiogenesis is a crucial step in tumor progression, including melanoma, making anti-angiogenic strategies a widely explored treatment approach. However, both innate and acquired resistance to these therapies suggest that this approach may need re-evaluation. Nanoparticles have gained attention for their potential to enhance drug delivery and retention within tumors via the bloodstream. However, the in vitro screening of nanoparticles is limited by the inability of preclinical models to replicate the complex tumor microenvironment, especially the blood supply. Here, it is demonstrated that melanoma cells embedded in Matrigel spheroids can engraft in and be vascularized by the chorioallantoic membrane (CAM) of fertilized chicken eggs. This model allows for the assessment of nanoparticle toxicity and accumulation in tumor spheroids, as well as functional effects such as angiogenesis. Cerium oxide nanoparticles (nanoceria) and their surface functionalized derivatives are widely explored for biomedical applications due to their ability to modulate oxidative stress and angiogenesis. Here, it is observed that heparin functionalized nanoceria penetrate melanoma spheroids in the CAM and promote spheroid vascularization to a greater extent than nanoceria alone. This study aids in the development of preclinical cancer models for nanoparticle screening and provides new insight into the interplay between nanoparticle surface coatings and biological effects.
Keywords: angiogenesis; cerium oxide; melanoma; nanoparticle; tumor spheroid.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

