Nanoparticle-Driven Skeletal Muscle Repair and Regeneration Through Macrophage-Muscle Stem Cell Interaction
- PMID: 40109124
- PMCID: PMC12051746
- DOI: 10.1002/smll.202412611
Nanoparticle-Driven Skeletal Muscle Repair and Regeneration Through Macrophage-Muscle Stem Cell Interaction
Abstract
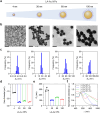
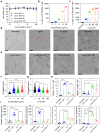
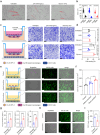
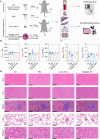
Macrophages are key innate immune cells in the muscle environment of sarcopenia patients, significantly influencing muscle stem cell (MuSC) proliferation and differentiation. However, prolonged activation of macrophages can hinder muscle recovery. In this study, it synthesizes lipoic acid-modified gold nanoparticles (LA-Au NPs) of varying sizes to evaluate their biocompatibility and immunomodulatory effects. The findings demonstrate that LA-Au NPs exhibit excellent biocompatibility with macrophages and promoted M2 polarization in a size-dependent manner. Mechanistically, LA-Au NPs facilitated metabolic reprogramming in macrophages by enhancing lysosomal autophagy and mitochondrial oxidative phosphorylation. Furthermore, macrophages are shown to chemotax toward MuSCs, regulating their proliferation via the chemokine system, inhibiting MuSC apoptosis, and enhancing differentiation under inflammatory conditions. In vivo studies have confirmed the safety and efficacy of LA-Au NPs in sarcopenia mice. To further enhance the effectiveness of LA-Au NPs, it investigates a delivery strategy that involves preconditioning macrophages with LA-Au NPs (Mac@Au NPs). Compared to the direct injection of LA-Au NPs, Mac@Au NPs demonstrate significantly greater benefits for muscle repair. This highlights the potential of macrophage therapy as a promising strategy for effective muscle regeneration and therapeutic intervention in sarcopenia.
Keywords: gold nanoparticles; immunometabolism; macrophages; muscle stem cells; skeletal muscle regeneration.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Gold nanoparticles modulate macrophage polarization to promote skeletal muscle regeneration.Mater Today Bio. 2025 Mar 11;32:101653. doi: 10.1016/j.mtbio.2025.101653. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40151803 Free PMC article. Review.
-
Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice.Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):10648-10653. doi: 10.1073/pnas.1806908115. Epub 2018 Oct 1. Proc Natl Acad Sci U S A. 2018. PMID: 30275293 Free PMC article.
-
Effect of silica and gold nanoparticles on macrophage proliferation, activation markers, cytokine production, and phagocytosis in vitro.Int J Nanomedicine. 2014 Dec 24;10:183-206. doi: 10.2147/IJN.S72580. eCollection 2015. Int J Nanomedicine. 2014. PMID: 25565813 Free PMC article.
-
Accelerated Bone Regeneration by Gold-Nanoparticle-Loaded Mesoporous Silica through Stimulating Immunomodulation.ACS Appl Mater Interfaces. 2019 Nov 6;11(44):41758-41769. doi: 10.1021/acsami.9b16848. Epub 2019 Oct 28. ACS Appl Mater Interfaces. 2019. PMID: 31610117
-
Metabolic regulation of macrophages during tissue repair: insights from skeletal muscle regeneration.FEBS Lett. 2017 Oct;591(19):3007-3021. doi: 10.1002/1873-3468.12703. Epub 2017 Jun 17. FEBS Lett. 2017. PMID: 28555751 Review.
Cited by
-
Perspectives on mitochondrial dysfunction in the regeneration of aging skeletal muscle.Cell Mol Biol Lett. 2025 Jul 28;30(1):94. doi: 10.1186/s11658-025-00771-1. Cell Mol Biol Lett. 2025. PMID: 40721743 Free PMC article. Review.
References
-
- Sayer A. A., Cooper R., Arai H., Cawthon P. M., Essomba M.‐J. N., Fielding R. A., Grounds M. D., Witham M. D., Cruz‐Jentoft A. J., Nature reviews. Disease primers 2024, 10, 68. - PubMed
-
- Cruz‐Jentoft A. J., Sayer A. A., Lancet 2019, 393, 2636. - PubMed
-
- Tuttle C. S. L., Thang L. A. N., Maier A. B., Ageing Res. Rev. 2020, 64, 101185. - PubMed
-
- Livshits G., Kalinkovich A., Ageing Res. Rev. 2023, 86, 101852. - PubMed
MeSH terms
Substances
Grants and funding
- JYY2023-11/Beijing Municipal Public Welfare Development and Reform Pilot Project for Medical Research Institutes
- NO.025/Youth Beijing Scholar Program
- CFH 2024-1-1121/Capital's Funds for Health Improvement and Research
- 7244287/Beijing Natural Science Foundation
- BJRITO-RDP/Beijing Municipal Health Commission
LinkOut - more resources
Full Text Sources
Medical

