Investigating Neotenic and Metamorphic Axolotl Brain Complexity: A Stereological and Immunohistochemical Perspective
- PMID: 40109227
- PMCID: PMC11923732
- DOI: 10.1002/cne.70031
Investigating Neotenic and Metamorphic Axolotl Brain Complexity: A Stereological and Immunohistochemical Perspective
Abstract
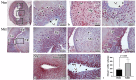
The ability of certain tetrapods, such as amphibians, to regenerate complex structures, such as organs or limbs, is well-established, though this capacity varies significantly across species, with humans exhibiting limited regenerative potential. Ependymoglia cells in the ventricular region of the brain are known to exhibit proliferative properties during homeostasis and damage and to perform stem cell functions. This study investigated changes occurring in neurons and glia in the central nervous system following metamorphosis in axolotls. Morphological alterations in brain tissue, newly formed neurons, and cellular organizations in different brain regions were assessed using stereological and immunohistochemical methods, as well as light and electron microscopy. Interestingly, we observe no statistically significant difference in total neuron numbers in the telencephalon region between neotenic and metamorphic axolotls. However, the proliferation index and the numbers of cells expressing NeuN were significantly higher in metamorphic axolotls. Furthermore, structural changes in neuronal nuclei and myelin sheath organization were determined at the light and electron microscopic levels post-metamorphosis. Ultrastructural analyses revealed a change in chromatin organization from euchromatic to heterochromatic in neurons after metamorphosis, and morphological changes were also demonstrated in myelinated nerve fibers in the telencephalon. Additionally, mucopolysaccharide-containing secretory sacs were also identified on the apical surfaces of a subgroup of ependymoglia cells located in the lateral ventricle wall. Overall, this study sheds useful light on the intricate changes occurring in the central nervous system during metamorphosis in axolotls and provides valuable insights into the mechanisms underlying these processes.
Keywords: RRID:AGSC_100A; axolotl; ependymoglia; metamorphosis; stereology; telencephalon.
© 2025 The Author(s). The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
A histological atlas of the tissues and organs of neotenic and metamorphosed axolotl.Acta Histochem. 2016 Sep;118(7):746-759. doi: 10.1016/j.acthis.2016.07.006. Epub 2016 Jul 18. Acta Histochem. 2016. PMID: 27436816
-
Effect of induced metamorphosis on the immune system of the axolotl, Ambystoma mexicanum.Gen Comp Endocrinol. 1995 Mar;97(3):308-19. doi: 10.1006/gcen.1995.1031. Gen Comp Endocrinol. 1995. PMID: 7789746
-
Comparison of protein expression profile of limb regeneration between neotenic and metamorphic axolotl.Biochem Biophys Res Commun. 2020 Feb 5;522(2):428-434. doi: 10.1016/j.bbrc.2019.11.118. Epub 2019 Nov 22. Biochem Biophys Res Commun. 2020. PMID: 31767146
-
Forever young: Endocrinology of paedomorphosis in the Mexican axolotl (Ambystoma mexicanum).Gen Comp Endocrinol. 2018 Sep 15;266:194-201. doi: 10.1016/j.ygcen.2018.05.016. Epub 2018 May 16. Gen Comp Endocrinol. 2018. PMID: 29777689 Review.
-
Organ regeneration evolved in fish and amphibians in relation to metamorphosis: Speculations on a post-embryonic developmental process lost in amniotes after the water to land transition.Ann Anat. 2019 Mar;222:114-119. doi: 10.1016/j.aanat.2018.12.005. Epub 2018 Dec 20. Ann Anat. 2019. PMID: 30580055 Review.
References
-
- Aoki, C. , and Erisir A.. 2014. “Experience‐Dependent Synaptic Plasticity in the Developing Cerebral Cortex.” In Synapse: Structure and Function, edited by Pickel V. and Segal M., 397–445. Academic Press. 10.1016/B978-0-12-418675-0.00013-4. - DOI
-
- Bancroft, J. D. , and Gamble M.. 2008. Theory and Practice of Histological Techniques. Churchill Livingstone. https://www.sciencedirect.com/book/9780443102790/theory‐and‐practice‐of‐....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

