The Triple Combination of Meropenem, Avibactam, and a Metallo-β-Lactamase Inhibitor Optimizes Antibacterial Coverage Against Different β-Lactamase Producers
- PMID: 40109291
- PMCID: PMC11913740
- DOI: 10.1016/j.eng.2024.02.010
The Triple Combination of Meropenem, Avibactam, and a Metallo-β-Lactamase Inhibitor Optimizes Antibacterial Coverage Against Different β-Lactamase Producers
Abstract
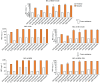
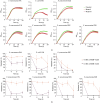
This work explores the potential of a triple combination of meropenem (MEM), a novel metallo-β-lactamase (MBL) inhibitor (indole-2-carboxylate 58 (InC58)), and a serine-β-lactamase (SBL) inhibitor (avibactam (AVI)) for broad-spectrum activity against carbapenemase-producing bacteria. A diverse panel comprising MBL- and SBL-producing strains was used for susceptibility testing of the triple combination using the agar dilution method. The frequency of resistance (FoR) to MEM combined with InC58 was investigated. Mutants were sequenced and tested for cross resistance, fitness, and the stability of the resistance phenotype. Compared with the double combinations of MEM plus an SBL or MBL inhibitor, the triple combination extended the spectrum of activity to most of the isolates bearing SBLs (oxacillinase-48 (OXA-48) and Klebsiella pneumoniae carbapenemase-2 (KPC-2)) and MBLs (New Delhi metallo-β-lactamases (NDMs)), although it was not effective against Verona integron-encoded metallo-β-lactamase (VIM)-carrying Pseudomonas aeruginosa (P. aeruginosa) and OXA-23-carrying Acinetobacter baumannii (A. baumannii). The FoR to MEM plus InC58 ranged from 2.22 × 10-7 to 1.13 × 10-6. The resistance correlated with mutations to ompC and comR, affecting porin C and copper permeability, respectively. The mutants manifested a fitness cost, a decreased level of resistance during passage without antibiotic pressure, and cross resistance to another carbapenem (imipenem) and a β-lactamase inhibitor (taniborbactam). In conclusion, compared with the dual combinations, the triple combination of MEM with InC58 and AVI showed a much wider spectrum of activity against different carbapenemase-producing bacteria, revealing a new strategy to combat β-lactamase-mediated antimicrobial resistance.
Keywords: Antimicrobial resistance; Avibactam; Carbapenemase; Meropenem; Metallo/serine-β-lactamase inhibitor.
© 2024 THE AUTHORS.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
