Evaluating oxidative stress targeting treatments in in vitro models of placental stress relevant to preeclampsia
- PMID: 40109359
- PMCID: PMC11920713
- DOI: 10.3389/fcell.2025.1539496
Evaluating oxidative stress targeting treatments in in vitro models of placental stress relevant to preeclampsia
Abstract
Background: Preeclampsia is a complex pregnancy disorder characterized by the new onset of hypertension and organ dysfunction, often leading to significant maternal and fetal morbidity and mortality. Placental dysfunction is a hallmark feature of preeclampsia, which is often caused by inappropriate trophoblast cell function in association with oxidative stress, inflammation and/or pathological hypoxia. This study explores the role of oxidative stress in trophoblast cell-based models mimicking the preeclamptic placenta and evaluates potential therapeutic strategies targeting these mechanisms.
Methods: Uric acid (UA) and malondialdehyde (MDA) concentrations were measured in human plasma from women with preeclampsia (n = 24) or normotensive controls (n = 14) using colorimetric assays. Custom-made first trimester trophoblast cell line, ACH-3P, was exposed to various preeclampsia-like stimuli including hypoxia mimetic (dimethyloxalylglycine or DMOG, 1 mM), inflammation (tumour necrosis factor or TNF-α, 10 ng/mL) or mitochondria dysfunction agent, (Rhodamine-6G or Rho-6G, 1 μg/mL), ± aspirin (0.5 mM), metformin (0.5 mM), AD-01 (100 nM) or resveratrol (15 µM), for 48 h. Following treatments, UA/MDA, proliferation (MTT), wound scratch and cytometric bead, assays, were performed.
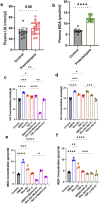
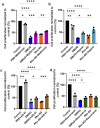
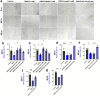
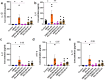
Results: Overall, MDA plasma concentration was increased in the preeclampsia group compared to healthy controls (p < 0.001) whereas UA showed a trend towards an increase (p = 0.06); when adjusted for differences in gestational age at blood sampling, MDA remained (p < 0.001) whereas UA became (p = 0.03) significantly correlated with preeclampsia. Our 2D first trimester trophoblast cell-based in vitro model of placental stress as observed in preeclampsia, mimicked the increase in UA concentration following treatment with DMOG (p < 0.0001), TNF-α (p < 0.05) or Rho-6G (p < 0.001) whereas MDA cell concentration increased only in the presence of DMOG (p < 0.0001) or Rho-6G (p < 0.001). Metformin was able to abrogate DMOG- (p < 0.01), Rho-6G- (p < 0.0001) or TNF-α- (p < 0.01) induced increase in UA, or DMOG- (p < 0.0001) or TNF-α- (p < 0.05)induced increase in MDA. AD-01 abrogated UA or MDA increase in the presence of TNF-α (p < 0.001) or Rho-6G (p < 0.001)/DMOG (p < 0.0001), respectively. The preeclampsia-like stimuli also mimicked adverse impact on trophoblast cell proliferation, migration and inflammation, most of which were restored with either aspirin, metformin, resveratrol, or AD-01 (p < 0.05).
Conclusion: Our 2D in vitro models recapitulate the response of the first trimester trophoblast cells to preeclampsia-like stresses, modelling inappropriate placental development, and demonstrate therapeutic potential of repurposed treatments.
Keywords: aspirin; metformin; oxidative stress; placenta; preeclampsia; pregnancy; resveratrol; trophoblast cells.
Copyright © 2025 Afrose, Johansen, Nikolic, Karadzov Orlic, Mikovic, Stefanovic, Cakic, Hansbro and McClements.
Conflict of interest statement
LM is an inventor on FKBPL-related patents for prediction and diagnosis of preeclampsia. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- ACOG (2019). Obstet. Gynecol. 133 (1), 1. 10.1097/AOG.0000000000003018 - DOI
-
- Afshari J. T., Ghomian N., Shameli A., Shakeri M., Fahmidehkar M., Mahajer E., et al. (2005). Determination of interleukin-6 and tumor necrosis factor-alpha concentrations in Iranian-khorasanian patients with preeclampsia. BMC Pregnancy Childbirth 5 (1), 14. 10.1186/1471-2393-5-14 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

