Regulatory pathways and guidelines for nanotechnology-enabled health products: a comparative review of EU and US frameworks
- PMID: 40109724
- PMCID: PMC11919859
- DOI: 10.3389/fmed.2025.1544393
Regulatory pathways and guidelines for nanotechnology-enabled health products: a comparative review of EU and US frameworks
Abstract
The integration of nanotechnology into healthcare has introduced Nanotechnology-Enabled Health Products (NHPs), promising revolutionary advancements in medical treatments and diagnostics. Despite their potential, the regulatory navigation for these products remains complex and often lagging, creating barriers to their clinical application. This review article focuses on dissecting the regulatory landscape for NHPs, particularly in the European Union and the United States, to identify applicable requirements and the main regulatory guidelines currently available for meeting regulatory expectations.
Keywords: European Union; United States; healthcare innovation; medical regulations; nanotechnology-enabled health products; regulatory pathways.
Copyright © 2025 Rodríguez-Gómez, Monferrer, Penon and Rivera-Gil.
Conflict of interest statement
FRG, OP, and DM were employed by company Asphalion SL. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
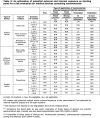
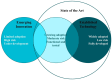
References
Publication types
LinkOut - more resources
Full Text Sources

