Research progress on the role of decorin in the development of oral mucosal carcinogenesis
- PMID: 40109852
- PMCID: PMC11915041
- DOI: 10.32604/or.2024.053119
Research progress on the role of decorin in the development of oral mucosal carcinogenesis
Abstract
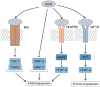
Decorin (DCN) is primarily found in the connective tissues of various parts of the body, including the lungs, kidneys, bone tissue, aorta, and tendons. It is an important component of the extracellular matrix (ECM) and belongs to the class I small leucine-rich proteoglycans family. DCN is increasingly attracting attention due to its significant role in tumors, fibrotic diseases, and the regulation of vascular formation. Moreover, its anti-tumor properties have positioned it as a promising biomarker in the fight against cancer. Numerous studies have confirmed that DCN can exert inhibitory effects in various solid tumors, particularly in oral squamous cell carcinoma (OSCC), by activating its downstream pathways through binding with the epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition (MET) receptor, or by stabilizing and enhancing the expression of the tumor suppressor gene p53 to mediate apoptosis in cancer cells that have undergone mutation. The occurrence of OSCC is a continuous and dynamic process, encompassing the transition from normal mucosa to oral potentially malignant disorders (OPMDs), and further progressing from OPMDs to the malignant transformation into OSCC. We have found that DCN may exhibit a bidirectional effect in the progression of oral mucosal carcinogenesis, showing a trend of initial elevation followed by a decline, which decreases with the differentiation of OSCC. In OPMDs, DCN exhibits high expression and may be associated with malignant transformation, possibly linked to the increased expression of P53 in OPMDs. In OSCC, the expression of DCN is reduced, which can impact OSCC angiogenesis, and inhibit tumor cell proliferation, migration, and invasion capabilities, serving as a potential marker for predicting adverse prognosis in OSCC patients. This article reviews the current research status of DCN, covering its molecular structure, properties, and involvement in the onset and progression of oral mucosal carcinogenesis. It elucidates DCN's role in this process and aims to offer insights for future investigations into its mechanism of action in oral mucosal carcinogenesis and its potential application in the early diagnosis and treatment of OSCC.
Keywords: Decorin (DCN); Oral erythroplakia (OEL); Oral leukoplakia (OLK); Oral lichen planus (OLP); Oral potentially malignant disorders (OPMDs); Oral squamous cell carcinoma (OSCC); Oral submucous fibrosis.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest to report regarding the present study.
Figures



References
-
- Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, Gonzalez-Moles MA, Kerr AR, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO collaborating centre for oral cancer. Oral Dis. 2021;27(8):1862–80; - PubMed
-
- Iocca O, Sollecito TP, Alawi F, Weinstein GS, Newman JG, De Virgilio A, et al. Potentially malignant disorders of the oral cavity and oral dysplasia: a systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck. 2020;42(3):539–55; - PubMed
-
- Warnakulasuriya S, Ariyawardana A. Malignant transformation of oral leukoplakia: a systematic review of observational studies. J Oral Pathol Med. 2016;45(3):155–66; - PubMed
-
- Wang X, Xu J, Wang L, Liu C, Wang H. The role of cigarette smoking and alcohol consumption in the differentiation of oral squamous cell carcinoma for the males in China. J Cancer Res Ther. 2015;11(1):141–5; - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
