Regioselective cyclocondensations with thiobarbituric acid: spirocyclic and azocine products, X-ray characterization, and antioxidant evaluation
- PMID: 40109931
- PMCID: PMC11921768
- DOI: 10.1039/d4ra07966c
Regioselective cyclocondensations with thiobarbituric acid: spirocyclic and azocine products, X-ray characterization, and antioxidant evaluation
Abstract
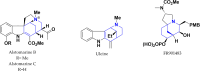
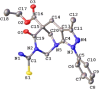
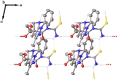
Multicomponent cyclocondensations of 5-amino-3-methyl-1-phenyl-1H-pyrazole (AMPZ), thiobarbituric acid, and p-formaldehyde under conventional thermal heating or ultrasonic irradiation were studied. Treatment of the reaction mixture in ethanol in an ultrasonic bath for 3 h produced azocine compound 4b, while the same mixture in ethanol under reflux conditions for 15 h produced spiro compound 4a. This work encompasses intricate experimental details, X-ray diffraction measurements, and multifaceted computational analyses employing methods such as the density functional theory and Hirshfeld surface analysis. Crystallographic investigations revealed the molecular structure of the compound and clarified its interactions involving hydrogen bonds and weak intermolecular forces. This article describes the synthesis and characterization of a novel spirocyclic compound. The study also evaluated the antioxidant potential in vitro using the DPPH and ABTS methods. The results showed that these compounds showed the best free radical scavenging ability, even in very small amounts, and that even at very low concentrations, these compounds showed excellent radical scavenging potential. Surprisingly, these compounds exhibited strong (ABTS+) radical scavenging activities, mainly attributed to the HAT mechanism, indicating their potential as therapeutic agents. Facile multipurpose, three-component selective procedures for new spiroheterocycles have been proposed, presenting intriguing perspectives in the field of medicine, particularly in the field of antioxidants. The geometric values of the computationally optimized structure were calculated using the density functional theory in LC-BLYP/6-31(d), aligned with the X-ray diffraction data, reinforcing the precision of our findings.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






Similar articles
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
-
Catalyst-Free, Room-Temperature Accessible Regioselective Synthesis of Spiroquinolines and Their Antioxidant Study.ACS Omega. 2022 Dec 19;8(1):444-456. doi: 10.1021/acsomega.2c05020. eCollection 2023 Jan 10. ACS Omega. 2022. PMID: 36643529 Free PMC article.
-
Synthesis, structure elucidation, SC-XRD/DFT, molecular modelling simulations and DNA binding studies of 3,5-diphenyl-4,5-dihydro-1H-pyrazole chalcones.J Biomol Struct Dyn. 2025 Mar;43(4):1831-1846. doi: 10.1080/07391102.2023.2293260. Epub 2023 Dec 12. J Biomol Struct Dyn. 2025. PMID: 38084878
-
Spectroscopic, single crystal X-ray, Hirshfeld, in vitro and in silico biological evaluation of a new series of potent thiazole nucleus integrated with pyrazoline scaffolds.Spectrochim Acta A Mol Biomol Spectrosc. 2017 Mar 5;174:254-271. doi: 10.1016/j.saa.2016.11.046. Epub 2016 Nov 28. Spectrochim Acta A Mol Biomol Spectrosc. 2017. PMID: 27936436
-
Synthesis, characterization, computational, antioxidant and fluorescence properties of novel 1,3,5-trimesic hydrazones derivatives.Heliyon. 2021 Sep 25;7(9):e08074. doi: 10.1016/j.heliyon.2021.e08074. eCollection 2021 Sep. Heliyon. 2021. PMID: 34622075 Free PMC article. Review.
References
-
- Lee S. Sperry J. Bioorg. Med. Chem. 2022;54:116560. - PubMed
-
- Natural Products, ed. K. G. Ramawat and J.-M. Mérillon, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013
-
- Zhuravleva O. I. Afiyatullov S. S. Denisenko V. A. Ermakova S. P. Slinkina N. N. Dmitrenok P. S. Kim N. Y. Phytochemistry. 2012;80:123–131. - PubMed
-
- Nakamura H. Deng S. Kobayashi J. Ohizumi Y. Tomotake Y. Matsuzaki T. Hirata Y. Tetrahedron Lett. 1987;28:621–624.
-
- Ndongo J. T. Shaaban M. Mbing J. N. Bikobo D. N. Atchadé A. D. T. Pegnyemb D. E. Laatsch H. Phytochemistry. 2010;71:1872–1878. - PubMed
LinkOut - more resources
Full Text Sources

