Effect of Bacillus subtilis isolated from yaks on D-galactose-induced oxidative stress and hepatic damage in mice
- PMID: 40109966
- PMCID: PMC11920168
- DOI: 10.3389/fmicb.2025.1550556
Effect of Bacillus subtilis isolated from yaks on D-galactose-induced oxidative stress and hepatic damage in mice
Abstract
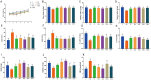
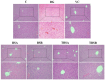
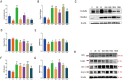
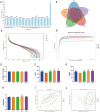
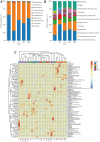
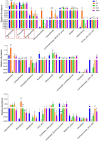
Acute hepatic injury is a severe condition that is always accompanied by oxidative stress and inflammation, seriously threatening the health of the host. Probiotics have been shown to be involved in the regulation of antioxidant system and gut microbiota activity, but studies on the effects of yak derived Bacillus subtilis (B. subtilis) on acute liver injury and oxidative stress remain scarce. Here, we aim to explore the ameliorative effects of B. subtilis isolated from yaks on oxidative stress and hepatic injury caused by D-galactose, as well as the underlying processes. Results indicated that B. subtilis administration, particularly the BS3, significantly mitigated hepatic damage induced by D-galactose in mice as evidenced by ameliorating liver tissue damage as well as decreasing ALT (p < 0.05) and AST (p < 0.05) levels. Additionally, the B. subtilis intervention was demonstrated to enhance the antioxidant system in D-galactose-exposed mice, as manifested by increased T-AOC and SOD, alongside a decrease in MDA levels (p < 0.05). Meanwhile, B. subtilis intervention could effectively mitigate oxidative damage via modulating the Keap1/Nrf2 signaling pathway. Importantly, B. subtilis exhibited a pronounced protective effect against D-galactose-induced intestinal barrier dysfunction through improving tight junction proteins. The gut microbiota results suggest that BS3 alters the abundance of some gut flora such as Firmicutes phylum and Oscillibacter and Lachnospiraceae_NK4A136 genera, which affects the composition of the gut microbiota and reverses the decrease in the microbial richness index in mice. In summary, these findings demonstrated that B. subtilis isolated from yaks serve as a promising candidate to ameliorate oxidative damage and hepatic injury. Meanwhile, the positive regulation effect of B. subtilis on gut microbiota and intestinal mucosal barrier may be one of its underlying mechanisms to alleviate oxidative stress and hepatic injury.
Keywords: Bacillus subtilis; Keap1/Nrf2 signaling pathway; gut microbiota; hepatic injury; oxidative stress; yak.
Copyright © 2025 Wang, Li, Zhang, Iqbal, Aabdin, Xu, Mo and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The ameliorative mechanism of Lactiplantibacillus plantarum NJAU-01 against D-galactose induced oxidative stress: a hepatic proteomics and gut microbiota analysis.Food Funct. 2024 Jun 4;15(11):6174-6188. doi: 10.1039/d4fo00406j. Food Funct. 2024. PMID: 38770619
-
Lactobacillus plantarum Y44 alleviates oxidative stress by regulating gut microbiota and colonic barrier function in Balb/C mice with subcutaneous d-galactose injection.Food Funct. 2021 Jan 7;12(1):373-386. doi: 10.1039/d0fo02794d. Epub 2020 Dec 16. Food Funct. 2021. PMID: 33325942
-
Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice.Microb Cell Fact. 2019 Jun 19;18(1):112. doi: 10.1186/s12934-019-1161-6. Microb Cell Fact. 2019. PMID: 31217027 Free PMC article.
-
Probiotic Bacillus Alleviates Oxidative Stress-Induced Liver Injury by Modulating Gut-Liver Axis in a Rat Model.Antioxidants (Basel). 2022 Jan 31;11(2):291. doi: 10.3390/antiox11020291. Antioxidants (Basel). 2022. PMID: 35204173 Free PMC article.
-
Antioxidant activity of selenium-enriched Chrysomyia megacephala (Fabricius) larvae powder and its impact on intestinal microflora in D-galactose induced aging mice.BMC Complement Med Ther. 2020 Aug 27;20(1):264. doi: 10.1186/s12906-020-03058-4. BMC Complement Med Ther. 2020. PMID: 32854685 Free PMC article.
References
-
- Chen H., Dong L., Chen X., Ding C., Hao M., Peng X., et al. . (2022). Anti-aging effect of phlorizin on D-galactose-induced aging in mice through antioxidant and anti-inflammatory activity, prevention of apoptosis, and regulation of the gut microbiota. Exp. Gerontol. 163:111769. doi: 10.1016/j.exger.2022.111769, PMID: - DOI - PubMed
-
- Chen Y., Yang B., Stanton C., Ross R. P., Zhao J., Zhang H., et al. . (2021). Bifidobacterium pseudocatenulatum ameliorates DSS-induced colitis by maintaining intestinal mechanical barrier, blocking proinflammatory cytokines, inhibiting TLR4/NF-kappaB signaling, and altering gut microbiota. J. Agric. Food Chem. 69, 1496–1512. doi: 10.1021/acs.jafc.0c06329, PMID: - DOI - PubMed
-
- Chiodini R. J., Dowd S. E., Chamberlin W. M., Galandiuk S., Davis B., Glassing A. (2015). Microbial population differentials between mucosal and submucosal intestinal tissues in advanced Crohn’s disease of the ileum. PLoS One 10:e0134382. doi: 10.1371/journal.pone.0134382, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

