Exploration of the mechanism and therapy of ovarian aging by targeting cellular senescence
- PMID: 40110109
- PMCID: PMC11916902
- DOI: 10.1093/lifemedi/lnaf004
Exploration of the mechanism and therapy of ovarian aging by targeting cellular senescence
Abstract
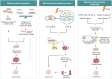
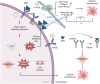
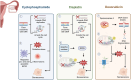
The ovary is a crucial gonadal organ that supports female reproductive and endocrine functions. Ovarian aging can result in decreased fertility and dysfunction across multiple organs. Research has demonstrated that cellular senescence in various cell types within the ovary can trigger a decline in ovarian function through distinct stress responses, resulting in ovarian aging. This review explores how cellular senescence may contribute to ovarian aging and reproductive failure. Additionally, we discuss the factors that cause ovarian cellular senescence, including the accumulation of advanced glycation end products, oxidative stress, mitochondrial dysfunction, DNA damage, telomere shortening, and exposure to chemotherapy. Furthermore, we discuss senescence in six distinct cell types, including oocytes, granulosa cells, ovarian theca cells, immune cells, ovarian surface epithelium, and ovarian endothelial cells, inside the ovary and explore their contribution to the accelerated ovarian aging. Lastly, we describe potential senotherapeutics for the treatment of ovarian aging and offer novel strategies for ovarian longevity.
Keywords: cellular senescence; granulosa cells; oocyte; ovarian aging; senotherapy.
© The Author(s) 2025. Published by Oxford University Press on behalf of Higher Education Press.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Figures


Similar articles
-
The impact of mitochondrial dysfunction on ovarian aging.J Transl Med. 2025 Feb 20;23(1):211. doi: 10.1186/s12967-025-06223-w. J Transl Med. 2025. PMID: 39980008 Free PMC article. Review.
-
The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions.Hum Reprod Update. 2022 Feb 28;28(2):172-189. doi: 10.1093/humupd/dmab038. Hum Reprod Update. 2022. PMID: 34918084 Free PMC article. Review.
-
Cellular senescence in the pathogenesis of ovarian dysfunction.J Obstet Gynaecol Res. 2024 May;50(5):800-808. doi: 10.1111/jog.15918. Epub 2024 Feb 27. J Obstet Gynaecol Res. 2024. PMID: 38412992 Review.
-
Ovarian aging: mechanisms and intervention strategies.Med Rev (2021). 2022 Nov 22;2(6):590-610. doi: 10.1515/mr-2022-0031. eCollection 2022 Dec. Med Rev (2021). 2022. PMID: 37724254 Free PMC article. Review.
-
Mechanisms of mitochondrial dysfunction in ovarian aging and potential interventions.Front Endocrinol (Lausanne). 2024 Apr 17;15:1361289. doi: 10.3389/fendo.2024.1361289. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38694941 Free PMC article. Review.
References
-
- Ata B, Seli E.. Strategies for controlled ovarian stimulation in the setting of ovarian aging. Semin Reprod Med 2015;33:436–48. - PubMed
-
- Minkin MJ. Menopause: hormones, lifestyle, and optimizing aging. Obstet Gynecol Clin North Am 2019;46:501–14. - PubMed
-
- Couzin-Frankel J. Reproductive biology. Faulty DNA repair linked to ovarian aging in mice and humans. Science 2013;339:749. - PubMed
-
- Sang Q, Ray PF, Wang L.. Understanding the genetics of human infertility. Science 2023;380:158–63. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
