Obesity promotes immunotherapy efficacy by up-regulating the glycolytic-mediated histone lactacylation modification of CD8+ T cells
- PMID: 40110127
- PMCID: PMC11920648
- DOI: 10.3389/fphar.2025.1533464
Obesity promotes immunotherapy efficacy by up-regulating the glycolytic-mediated histone lactacylation modification of CD8+ T cells
Abstract
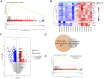
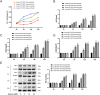
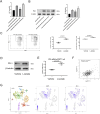
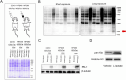
The response rate of immune checkpoint blockade (ICB) therapy for non-small-cell lung cancer (NSCLC) remains limited. Recent evidence suggests that obese cancer patients are more likely to benefit from ICB therapy, however, the specific mechanism needs further research. In this study, we found that anti-PD-1 therapy was more effective in obese NSCLC patients compared to normal weight patients and this was verified in mouse NSCLC model. Further bioinformatics analysis indicated that the glycolytic metabolism was markedly elevated in obese NSCLC patients. In vitro co-culture experiment showed that both increased glycolysis of tumor cells and external addition of lactate promoted T cell PD-1 expression. And, PD-1 upregulation was related to monocarboxylate transporter 1 (MCT1)-mediated lactate transport and subsequent lysine lactylation of histones in T cells. Based on the aforementioned data, our study contributes to better application of anti-PD-1 therapy in NSCLC.
Keywords: MCT1; NSCLC; PD-1; histone lactacylation; immunotherapy.
Copyright © 2025 Wang, Shi, Shi, Wang and Ai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., et al. (2018). Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391, 1023–1075. 10.1016/S0140-6736(17)33326-3 - DOI - PMC - PubMed
-
- Beltra J. C., Manne S., Abdel-Hakeem M. S., Kurachi M., Giles J. R., Chen Z., et al. (2020). Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52, 825–841. 10.1016/j.immuni.2020.04.014 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

