Amentoflavone protects against cisplatin-induced acute kidney injury by modulating Nrf2-mediated oxidative stress and ferroptosis and partially by activating Nrf2-dependent PANoptosis
- PMID: 40110131
- PMCID: PMC11919867
- DOI: 10.3389/fphar.2025.1508047
Amentoflavone protects against cisplatin-induced acute kidney injury by modulating Nrf2-mediated oxidative stress and ferroptosis and partially by activating Nrf2-dependent PANoptosis
Abstract
Background: Cisplatin is a widely used drug for the treatment of solid organ cancer, but its renal toxicity cannot be ignored. Amentoflavone (AME), a natural flavonoid compound, has remarkable pharmacological effects, including anti-inflammatory and antioxidative effects. The effect and mechanism of AME on cisplatin-induced acute kidney injury (CI-AKI) remain unclear.
Methods: We investigated the effect of AME on CI-AKI using the HK-2 cell line and C57BL/6 mice. Renal function, tissue damage, and molecular markers were assessed to explore the effects of AME on oxidative stress and cell death pathways.
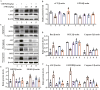
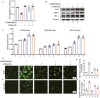
Results: In vitro, AME significantly suppressed the cytotoxic effects of cisplatin on HK-2 cells. Furthermore, AME significantly inhibited cisplatin-induced ferroptosis and PANoptosis (apoptosis, pyroptosis and necroptosis). In mice with acute kidney injury induced by a single intraperitoneal injection of cisplatin, the daily administration of AME during AKI effectively improved renal function and alleviated renal tubular injury, characterized by the normalization of blood urea nitrogen (BUN) and serum creatinine (SCr) levels; it also inhibited cisplatin-induced renal ferroptosis and PANoptosis. AME is a natural antioxidant that activates the Nrf2 antioxidant pathway both in vivo and in vitro. In Nrf2 knockout mice and knockdown cells, the protective effect of AME against cisplatin-induced nephrotoxicity disappeared. However, after Nrf2 knockout, the effect of AME on ferroptosis completely disappeared, and that on PANoptosis partially disappeared.
Conclusion: Amentoflavone has a protective effect on cisplatin-induced acute kidney injury via a mechanism related to the Nrf2-dependent antioxidant pathway and the regulation of ferroptosis and PANoptosis.
Keywords: CI-AKI; Nrf2; PANoptosis; amentoflavone; ferroptosis; oxidative stress.
Copyright © 2025 Zhang, Hu, Zhang and Ci.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources

