Artificial intelligence for the analysis of intracoronary optical coherence tomography images: a systematic review
- PMID: 40110224
- PMCID: PMC11914731
- DOI: 10.1093/ehjdh/ztaf005
Artificial intelligence for the analysis of intracoronary optical coherence tomography images: a systematic review
Abstract
Intracoronary optical coherence tomography (OCT) is a valuable tool for, among others, periprocedural guidance of percutaneous coronary revascularization and the assessment of stent failure. However, manual OCT image interpretation is challenging and time-consuming, which limits widespread clinical adoption. Automated analysis of OCT frames using artificial intelligence (AI) offers a potential solution. For example, AI can be employed for automated OCT image interpretation, plaque quantification, and clinical event prediction. Many AI models for these purposes have been proposed in recent years. However, these models have not been systematically evaluated in terms of model characteristics, performances, and bias. We performed a systematic review of AI models developed for OCT analysis to evaluate the trends and performances, including a systematic evaluation of potential sources of bias in model development and evaluation.
Keywords: Artificial intelligence; Coronary; Deep learning; Intravascular imaging; Optical coherence tomography; Systematic review.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: G.W.S. declares grants from Shockwave, Biosense-Webster, Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Vascular Dynamics, Pulnovo, V-wave, and PCORI (via Weill Cornell Medical Center); consulting fees from Cardiomech, Robocath, Daiichi Sankyo, Vectorious, Miracor, Apollo Therapeutics, Abbott, Cardiac Success, Occlutech, Millennia Biopharma, Remote Cardiac Enablement, Ablative Solutions, Valfix, Zoll, HeartFlow, Shockwave, Impulse Dynamics, Adona Medical, Oxitope, HighLife, Elixir, Elucid Bio, and Aria; speaker fee from Pulnovo, Medtronic, Amgen, Boehringer Ingelheim, and Abiomed; stocks for Cardiac Success, Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Valfix, and Xenter. N.H. declares grants from Abbott, Biosensors, Boston Scientific, and Medis Medical Imaging; speaker fee from Abbott and Teruma. E.K. declares grants from Abbott and Medtronic; speaker fee from Abbott; stocks for Electroducer and V3. J.E. declares speaker fee from Abbott. D.P. declares speaker fee from Abbott and GE Healthcare. G.G. declares grants from Abbott; consulting fees from Abbott, Infraredx, Panorama Scientific, Gentuity, InnovaHTS, and Terra Quantum; speaker fee from GE Medical, Nipro, and Abbott; payment for expert testimony from ConneX Biomedical; support for attending meetings from Abbott. S.M. declares grants from Abbott; consulting fees from NovoNordisk, Amgen, and Johnson and Johnson; participation on an advisory board in MK-0616 Coral Reef outcomes. N.P.-E. declares grants from Abbott Vascular; consulting fees from Abbott Vascular and Philips; speaker fee from Abbott Vascular, Philips, SIS Medical, and Novartis. R.M. declares consulting fees from Abbott Vascular, Boston Scientific, and Medtronic Inc.; speaker fee from Boston Scientific, Bayer, Daiichi Sankyo, Meril, Edwards LifeSciences, AMGEN, Biotronik, Biosensors, Abbott Vascular, Braun, and Philips. L.R. declares grants from Abbott, Boston Scientific, Biotronik, Infraredx, Sanofi, and Regeneron; consulting fees from Abbott, Canon, Gentuity, Medtronic, NovoNordisc, Occlutech, and Sanofi. B.v.G. declares stocks for Thirona. C.I.S. declares grants from Novartis and Boehringer Ingelheim; speaker fee from Novartis and Bayer. I.I. declares grants or contracts from Pie Medical Imaging BV, Esaote SpA, Dutch Science Foundation with participation of Philips Healthcare, Pie Medical Imaging, GE, Abbott, Heu, and IHI; support for attending meetings from SCCT; patents planned, issued, or pending from US patent 10,176,575; 10,395,366; 11,004,198; 10,699,407; and from US App. Patent US17/317,746; US16/911,323; US18/206,536. N.v.R. declares grants from Koninklijke Philips NV, Biotronik, Medtronic Inc, Abbott; speaker fee from MicroPort, Bayer AG, and RainMed Medical.
Figures


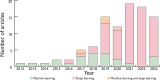

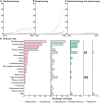

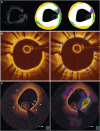
References
-
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. - PubMed
-
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023;44:3720–3826. - PubMed
-
- Holm NR, Andreasen LN, Neghabat O, Laanmets P, Kumsars I, Bennett J, et al. OCT or angiography guidance for PCI in complex bifurcation lesions. N Engl J Med 2023;389:1477–1487. - PubMed
-
- Stone GW, Christiansen EH, Ali ZA, Andreasen LN, Maehara A, Ahmad Y, et al. Intravascular imaging-guided coronary drug-eluting stent implantation: an updated network meta-analysis. Lancet 2024;403:824–837. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
