The impact of ivermectin on COVID-19 outcomes: a systematic review and meta-analysis
- PMID: 40110299
- PMCID: PMC11918548
- DOI: 10.1097/MS9.0000000000002762
The impact of ivermectin on COVID-19 outcomes: a systematic review and meta-analysis
Abstract
Background: The COVID-19 pandemic, resulting in approximately seven million deaths globally, underscores the urgency for effective treatments. Ivermectin, among several repurposed drugs, garnered interest due to its antiviral properties. However, conflicting evidence from observational studies and randomized controlled trials raised questions about its efficacy and safety.
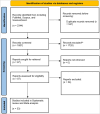
Method: This systematic review and meta-analysis followed MOOSE and PRISMA guidelines. Comprehensive searches were conducted in databases including Scopus, Embase, PubMed, and Web of Science up to April 2024. Data were extracted independently by two reviewers and analyzed using Comprehensive Meta-Analysis V3 software.
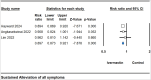
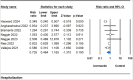
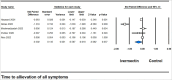
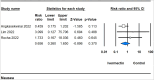
Results: Across 33 studies encompassing 15,376 participants, ivermectin showed no significant impact on critical outcomes such as mortality [risk ratio (RR) 0.911, 95% confidence intervals (CI) 0.732-1.135], mechanical ventilation (RR 0.727, 95% CI 0.521-1.016), polymerase chain reaction conversion (RR 1.024, 95% CI 0.936-1.120), ICU admissions (RR 0.712, 95% CI 0.274-1.850), or hospitalization rates (RR 0.735, 95% CI 0.464-1.165) compared to controls. However, it significantly reduced time to symptom alleviation (standardized mean difference -0.302, 95% CI -0.587 to -0.018) and sustained symptom relief (RR 0.897, 95% CI 0.873-0.921). Adverse event (AE) rates were similar between the ivermectin and control groups (RR 0.896, 95% CI 0.797-1.007). Meta-regression indicated older age and diabetes as predictors of AEs.
Conclusion: Despite its observed benefits in symptom management, ivermectin did not significantly influence critical clinical outcomes in COVID-19 patients. These findings highlight the importance of continued research to identify effective treatments for COVID-19, emphasizing the need for high-quality studies with robust methodology to inform clinical practice and public health policy effectively.
Keywords: COVID-19; ivermectin; meta-analysis; mortality; treatment efficacy.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Figures









References
-
- Coronavirus N. Situation report-1. Geneva: World Health Organization; 2020. 2019.
-
- Theoharides TC, Conti P. Dexamethasone for COVID-19? Not so fast. J Biol Regul Homeost Agents 2020;34:1241–43. - PubMed
-
- Raiteri A, Piscaglia F, Granito A, et al. . Tocilizumab: from rheumatic diseases to COVID-19. Curr Pharm Des 2021;27:1597–607. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
