Application of CRISPR-Cas System in Human Papillomavirus Detection Using Biosensor Devices and Point-of-Care Technologies
- PMID: 40110345
- PMCID: PMC11922499
- DOI: 10.34133/bmef.0114
Application of CRISPR-Cas System in Human Papillomavirus Detection Using Biosensor Devices and Point-of-Care Technologies
Abstract
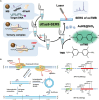
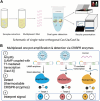
Human papillomavirus (HPV) is the most common virus for genital tract infections. Cervical cancer ranks as the fourth most prevalent cancer globally, with over 99% of cases in women attributed to HPV infection. This infection continues to pose an ongoing threat to public health. Therefore, the development of rapid, high-throughput, and sensitive HPV detection platforms is important, especially in regions with limited access to advanced medical resources. CRISPR-based biosensors, a promising new method for nucleic acid detection, are now rapidly and widely used in basic and applied research and have received much attention in recent years for HPV diagnosis and treatment. In this review, we discuss the mechanisms and functions of the CRISPR-Cas system, focusing on its applications in HPV diagnostics. The review covers CRISPR technologies such as CRISPR-Cas9, CRISPR-Cas12, and CRISPR-Cas13, along with nucleic acid amplification methods, CRISPR-based signal output systems, and point-of-care testing (POCT) strategies. This comprehensive overview highlights the versatility and potential of CRISPR technologies in HPV detection. We also discuss the numerous CRISPR biosensors developed since the introduction of CRISPR to detect HPV. Finally, we discuss some of the challenges faced in HPV detection by the CRISPR-Cas system.
Copyright © 2025 Chang He et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures





Similar articles
-
CRISPR/Cas-based nucleic acid detection strategies: Trends and challenges.Heliyon. 2024 Feb 14;10(4):e26179. doi: 10.1016/j.heliyon.2024.e26179. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38390187 Free PMC article. Review.
-
Detection of HPV 16 and 18 L1 genes by a nucleic acid amplification-free electrochemical biosensor powered by CRISPR/Cas9.Bioelectrochemistry. 2025 Apr;162:108861. doi: 10.1016/j.bioelechem.2024.108861. Epub 2024 Nov 23. Bioelectrochemistry. 2025. PMID: 39608317
-
CRISPR-Cas systems for diagnosing infectious diseases.Methods. 2022 Jul;203:431-446. doi: 10.1016/j.ymeth.2021.04.007. Epub 2021 Apr 9. Methods. 2022. PMID: 33839288 Free PMC article. Review.
-
CRISPR-cas technology: A key approach for SARS-CoV-2 detection.Front Bioeng Biotechnol. 2023 Apr 12;11:1158672. doi: 10.3389/fbioe.2023.1158672. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37214290 Free PMC article. Review.
-
Advancements in CRISPR-Based Biosensing for Next-Gen Point of Care Diagnostic Application.Biosensors (Basel). 2023 Jan 29;13(2):202. doi: 10.3390/bios13020202. Biosensors (Basel). 2023. PMID: 36831968 Free PMC article. Review.
Cited by
-
Emerging implications of N6-methyladenosine in prostate cancer progression and treatment.Cell Death Discov. 2025 Aug 19;11(1):391. doi: 10.1038/s41420-025-02680-w. Cell Death Discov. 2025. PMID: 40830264 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Jin J. HPV infection and cancer. JAMA. 2018;319(10):1058. - PubMed
-
- Plotzker RE, Vaidya A, Pokharel U, Stier EA. Sexually transmitted human papillomavirus: Update in epidemiology, prevention, and management. Infect Dis Clin N Am. 2023;37(2):289–310. - PubMed
-
- Forman D, Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, et al. . Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

