Altered Gut Microbiota Contributes to Acute-Respiratory-Distress-Syndrome-Related Depression through Microglial Neuroinflammation
- PMID: 40110391
- PMCID: PMC11919824
- DOI: 10.34133/research.0636
Altered Gut Microbiota Contributes to Acute-Respiratory-Distress-Syndrome-Related Depression through Microglial Neuroinflammation
Abstract
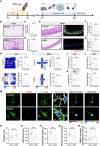
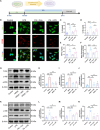
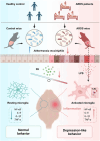
Acute respiratory distress syndrome (ARDS) survivors often suffer from long-term psychiatric disorders such as depression, but the underlying mechanisms remain unclear. Here, we found marked alterations in the composition of gut microbiota in both ARDS patients and mouse models. We investigated the role of one of the dramatically changed bacteria-Akkermansia muciniphila (AKK), whose abundance was negatively correlated with depression phenotypes in both ARDS patients and ARDS mouse models. Specifically, while fecal transplantation from ARDS patients into naive mice led to depressive-like behaviors, microglial activation, and intestinal barrier destruction, colonization of AKK or oral administration of its metabolite-propionic acid-alleviated these deficits in ARDS mice. Mechanistically, AKK and propionic acid decreased microglial activation and neuronal inflammation through inhibiting the Toll-like receptor 4/nuclear factor κB signaling pathway. Together, these results reveal a microbiota-dependent mechanism for ARDS-related depression and provide insight for developing a novel preventative strategy for ARDS-related psychiatric symptoms.
Copyright © 2025 Bowen Zhu et al.
Figures




References
-
- Bos LDJ, Ware LB. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet. 2022;400(10358):1145–1156. - PubMed
-
- Huang M, Parker AM, Bienvenu OJ, Dinglas VD, Colantuoni E, Hopkins RO, Needham DM, National Institutes of Health, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Psychiatric symptoms in acute respiratory distress syndrome survivors: A 1-year national multicenter study. Crit Care Med. 2016;44(5):954–965. - PMC - PubMed
LinkOut - more resources
Full Text Sources

