Long-term Safety and Effectiveness of Rezafungin Treatment in Candidemia and Invasive Candidiasis: Results From an Early Access Program in Italy and Germany
- PMID: 40110419
- PMCID: PMC11920862
- DOI: 10.1093/ofid/ofaf034
Long-term Safety and Effectiveness of Rezafungin Treatment in Candidemia and Invasive Candidiasis: Results From an Early Access Program in Italy and Germany
Abstract
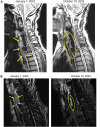
Outcomes are reported for 6 adults receiving rezafungin for chronic, hard-to-treat, invasive candidiasis (including Candida parapsilosis) during an early access program. Rezafungin was well tolerated and administered via once-weekly outpatient intravenous infusion for up to 39 weeks during the program, enabling hospital discharge and replacing daily antifungal infusions.
Keywords: candidemia; early access program; echinocandin; invasive candidiasis; rezafungin.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. Filippo Trapani, Giulio Viceconte, Valentina Morena, Giovanni Mori, Elham Khatamzas, and Britta Kölking have no relevant financial disclosures. Giusy Tiseo received speaker honoraria for educational meetings by Shionogi and honoraria for participation in a scientific advisory board by MSD. The study was funded by Mundipharma Research Limited.
Figures
References
-
- Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infectn 2019; 25:1200–12. - PubMed
-
- Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect 2019; 25:792–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

