Abnormal Synchronization Between Cortical Delta Power and Ripples in Hippocampal Sclerosis
- PMID: 40110652
- PMCID: PMC12093325
- DOI: 10.1002/acn3.70032
Abnormal Synchronization Between Cortical Delta Power and Ripples in Hippocampal Sclerosis
Abstract
Objective: Discriminating between epileptogenic and physiological ripples in the hippocampus is important for identifying epileptogenic (EP) zones; however, distinguishing these ripples on the basis of their waveforms is difficult. We hypothesized that the nocturnal synchronization of hippocampal ripples and cortical delta power could be used to classify epileptogenic and physiological ripples in the hippocampus.
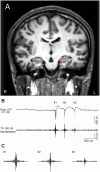
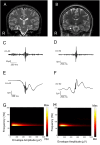
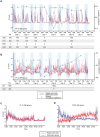
Methods: We enrolled 38 patients with electrodes implanted in the hippocampus or parahippocampal gyrus between April 2014 and March 2023 at our institution. We divided 11 patients (11 hippocampi) who were pathologically diagnosed with hippocampal sclerosis into the EP group and five patients (six hippocampi) with no epileptogenicity in the hippocampus into the nonepileptogenic (NE) group. Hippocampal ripples were detected using intracranial electroencephalography with hippocampal or parahippocampal electrodes. Cortical delta power (0.5-4 Hz) was assessed using cortical electrodes. The Pearson correlation coefficient between the ripple rates and cortical delta power (Corr-RD) was calculated on the basis of the intracranial electroencephalographic signals recorded each night.
Results: Although hippocampal ripples were similar among the EP and NE groups based on their waveforms and frequency properties, the Corr-RDs in the EP group (mean [standard deviation]: 0.20 [0.049]) were significantly lower than those in the NE group (0.67 [0.070]). On the basis of the minimum Corr-RDs, the two groups were classified with 94.1% accuracy.
Interpretation: Our results demonstrate that the Corr-RD is a biomarker of hippocampal epileptogenicity.
Keywords: delta band power; epileptogenicity; hippocampus; pathological ripple; sharp‐wave ripple.
© 2025 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Liu S., Sha Z., Sencer A., et al., “Exploring the Time‐Frequency Content of High Frequency Oscillations for Automated Identification of Seizure Onset Zone in Epilepsy,” Journal of Neural Engineering 13, no. 2 (2016): 26026. - PubMed
-
- González Otárula K. A., von Ellenrieder N., Cuello‐Oderiz C., Dubeau F., and Gotman J., “High‐Frequency Oscillation Networks and Surgical Outcome in Adult Focal Epilepsy,” Annals of Neurology 85, no. 4 (2019): 485–494. - PubMed
MeSH terms
Grants and funding
- JPMJER1801/Exploratory Research for Advanced Technology
- JP19dm020707/Japan Agency for Medical Research and Development
- JP19dm0307103/Japan Agency for Medical Research and Development
- JP19dm0307008/Japan Agency for Medical Research and Development
- JP24wm0625207/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Miscellaneous
