Toward molecular phenotyping of temporal lobe epilepsy by spatial omics
- PMID: 40110881
- PMCID: PMC12290997
- DOI: 10.1111/epi.18366
Toward molecular phenotyping of temporal lobe epilepsy by spatial omics
Abstract
Objective: In temporal lobe epilepsy (TLE), detection of the epileptogenic zone predicts a good surgical outcome. When submitted to 18F-fluorodeoxyglucose positron emission tomography (PET), some patients display lateralized, focal hypometabolism in the temporal lobe (PET+), whereas others appear normometabolic (PET-). However, the mechanism behind this metabolic difference remains unclear. This study aimed to identify differential molecular mechanisms in these patient subtypes.
Methods: Neocortical and hippocampal biopsies of TLE patients (n = 3 PET+, n = 3 PET-) and nonepileptic postmortem controls (n = 3) were analyzed for lipid distribution using mass spectrometry imaging (MSI). Laser capture microdissection of the neocortical gray matter and hippocampal cornu ammonis and dentate gyrus was guided by MSI-derived lipid profiles and histological annotations. Dissected areas were then subjected to liquid chromatography- tandem mass spectrometry-based label-free quantitative proteomic analysis.
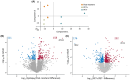
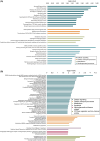
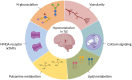
Results: MSI showed distinct lipid profiles, namely, phosphatidylserines were more abundant in PET+ samples in both the neocortex and hippocampus. Proteomic analysis showed significant differences between TLE and nonepileptic postmortem controls involving pathways in neuron excitability and neurotransmitter transporters, which were upregulated in TLE. Compared to PET-, all PET+ specimens displayed significantly dysregulated calcium signaling. Additionally, the neocortex of PET+ patients showed a shift from mitochondrial to cytosolic (cytoplasm of the cell) processes, whereas the hippocampus was characterized by a disruption of glycosylation and polyamine metabolism.
Significance: The applied spatial omics approach demonstrated localized molecular differences between metabolic subtypes of TLE patients. These findings may further specify these TLE subtypes and provide leads for targeted treatment.
Keywords: MALDI‐MSI; lipidomics; metabolism; proteomics; temporal lobe epilepsy.
© 2025 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Figures


References
-
- Alomar SA, Moshref RH, Moshref LH, Sabbagh AJ. Outcomes after laser interstitial thermal ablation for temporal lobe epilepsy: a systematic review and meta‐analysis. Neurosurg Rev. 2023;46:261. - PubMed
-
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal‐lobe epilepsy New England. J Med. 2001;345:311–318. - PubMed
-
- Haemels M, Van Weehaeghe D, Cleeren E, Dupont P, van Loon J, Theys T, et al. Predictive value of metabolic and perfusion changes outside the seizure onset zone for postoperative outcome in patients with refractory focal epilepsy. Acta Neurol Belg. 2022;122:325–335. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

