Effect of prevaccination blood and T-cell phenotypes on antibody responses to a COVID-19 mRNA vaccine
- PMID: 40110889
- PMCID: PMC12190804
- DOI: 10.1093/intimm/dxaf013
Effect of prevaccination blood and T-cell phenotypes on antibody responses to a COVID-19 mRNA vaccine
Abstract
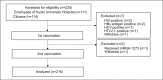
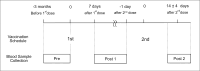
Despite the high effectiveness of the coronavirus disease 2019 (COVID-19) mRNA vaccines, both immunogenicity and reactogenicity show substantial interindividual variability. One key challenge is predicting high and low responders using easily measurable parameters. In this study, we performed multivariate linear regression analysis, which allows adjustment for confounding, to explore independent predictive factors for antibody responses. Using data from 216 healthy vaccinated donors aged 23-81 years, we evaluated baseline characteristics, prevaccination blood and T-cell phenotypes, and post-vaccination T-cell responses as variables, with anti-receptor-binding domain (RBD) immunoglobulin G (IgG) titers following two doses of BNT162b2 vaccination as the primary outcome. Consistent with previous reports, higher age, a history of allergic disease, and autoimmune disease were associated with lower peak IgG titers. Additionally, the frequencies of interferon-γ+ spike-specific CD4+ T cells (T-cell response) following the first vaccination strongly correlated with higher IgG responses, while those of pre-existing spike-reactive T cells showed no association with peak IgG titers. Furthermore, we identified lower percentages of naïve CD8+ T cells, lower hemoglobin levels, lower lymphocyte counts, and higher mean corpuscular volume as independent pre-vaccination predictors of lower peak IgG levels. Notably, the frequency of naïve CD8+ T cells showed a positive correlation with the peak IgG levels even in univariate analysis. These findings contribute to the individualized prediction of mRNA vaccine efficacy and may provide insights into the mechanisms underlying individual heterogeneity in immune responses.
Keywords: SARS-CoV-2; immunogenicity; multivariate linear regression analysis; prediction of vaccine response; vaccine efficacy.
© The Author(s) 2025. Published by Oxford University Press on behalf of The Japanese Society for Immunology.
Figures

References
-
- Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020;586:594–9. https://doi.org/ 10.1038/s41586-020-2814-7 - DOI - PubMed
-
- Collier DA, Ferreira IATM, Kotagiri P, et al. ; CITIID-NIHR BioResource COVID-19 Collaboration. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021;596:417–22. https://doi.org/ 10.1038/s41586-021-03739-1 - DOI - PMC - PubMed
-
- Arunachalam PS, Scott MKD, Hagan T, et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021;596:410–6. https://doi.org/ 10.1038/s41586-021-03791-x - DOI - PMC - PubMed
-
- Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med 2021;9:999–1009. https://doi.org/ 10.1016/S2213-2600(21)00220-4 - DOI - PMC - PubMed
-
- Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA Vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med 2021;174:1572–85. https://doi.org/ 10.7326/M21-1757 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- JP17gm5010005/Japan Agency for Medical Research and Development
- JP20fk0108265/Japan Agency for Medical Research and Development
- JP20fk0108454/Japan Agency for Medical Research and Development
- JP223fa627009/Japan Agency for Medical Research and Development
- JP24gm1810012/Japan Agency for Medical Research and Development
- iPS Cell Research Fund
- COVID-19 Private Fund to the Shinya Yamanaka Laboratory
- CiRA
- Kyoto University
- Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 21K15467/Japan Society for the Promotion of Science
- Kansai Economic Federation
- Sumitomo-Mitsui Trust Bank Fund for COVID-19 Research
- Takeda Science Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

