Midline Venous Catheter vs Peripherally Inserted Central Catheter for Intravenous Therapy: A Randomized Clinical Trial
- PMID: 40111366
- PMCID: PMC11926630
- DOI: 10.1001/jamanetworkopen.2025.1258
Midline Venous Catheter vs Peripherally Inserted Central Catheter for Intravenous Therapy: A Randomized Clinical Trial
Abstract
Importance: Peripherally inserted central catheters (PICCs) are frequently used for peripheral intravenous therapy (IVT) that could be administered through a peripheral midline venous catheter (MVC).
Objective: To assess the noninferiority of MVCs compared with PICCs as a reliable vascular access for peripheral IVT and blood draws for IVT that does not require a central VC.
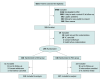
Design, setting, and participants: This randomized clinical trial was conducted in a single tertiary care center from September 2018 to March 2022. Participants were all consecutive adult patients who were referred for PICC and eligible for MVC. Patients likely to require a central VC (those in the critical care unit, those with kidney failure, or those requiring a multilumen VC) were excluded. Analyses were based on the evaluable population.
Interventions: Participants were randomized 1:1 to either MVC or PICC. For the MVC group, a 20-cm-long, 4F (French), single-lumen MVC without a valve was used without fluoroscopic assistance. For the PICC group, a 4F, single-lumen PICC without a valve was positioned under fluoroscopy at the cavoatrial junction.
Main outcomes and measures: The primary outcome was the percentage of patients without VC-related adverse events or dysfunctions requiring medical intervention during follow-up. A noninferiority test was performed to compare the proportion of adverse events or dysfunctions between the MVC and PICC groups. A noninferiority margin was set at 10% and a 5% 1-sided significance level.
Results: Of the 6821 patients referred to the tertiary care center for PICC insertion, 294 (180 males [61.2%]; median [IQR] age, 56.3 [38.2-66.7] years) were randomized to receive MVCs (n = 146) or PICCs (n = 148); 135 and 137 participants, respectively, were included in data analysis after exclusion of those who did not complete follow-up. Ninety of 135 patients (66.7%) in the MVC group and 128 of 137 (93.4%) in the PICC group were without VC-related adverse event or dysfunction. The noninferiority of MVC could not be demonstrated (P > .99 for noninferiority).
Conclusions and relevance: In this randomized clinical trial, MVCs were associated with a significantly higher percentage of patients with VC-related adverse events or dysfunctions and could not be demonstrated as a noninferior alternative to PICCs.
Trial registration: ClinicalTrials.gov Identifier: NCT03502980.
Conflict of interest statement
Figures

Comment in
References
-
- Chopra V, Flanders SA, Saint S, et al. ; Michigan Appropriateness Guide for Intravenouse Catheters (MAGIC) Panel . The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1-S40. doi:10.7326/M15-0744 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

