TP53 mutations and TET2 deficiency cooperate to drive leukemogenesis and establish an immunosuppressive environment
- PMID: 40111422
- PMCID: PMC12077897
- DOI: 10.1172/JCI184021
TP53 mutations and TET2 deficiency cooperate to drive leukemogenesis and establish an immunosuppressive environment
Abstract
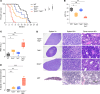
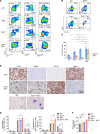
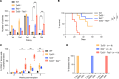
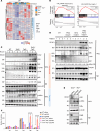
Mutations and deletions in TP53 are associated with adverse outcomes in patients with myeloid malignancies, and there is an urgent need for the development of improved therapies for TP53-mutant leukemias. Here, we identified mutations in TET2 as the most common co-occurring mutation in patients with TP53-mutant acute myeloid leukemia (AML). In mice, combined hematopoietic-specific deletion of TET2 and TP53 resulted in enhanced self-renewal compared with deletion of either gene alone. Tp53/Tet2 double-KO mice developed serially transplantable AML. Both mice and patients with AML with combined TET2/TP53 alterations upregulated innate immune signaling in malignant granulocyte-monocyte progenitors, which had leukemia-initiating capacity. A20 governs the leukemic maintenance by triggering aberrant noncanonical NF-κB signaling. Mice with Tp53/Tet2 loss had expansion of monocytic myeloid-derived suppressor cells (MDSCs), which impaired T cell proliferation and activation. Moreover, mice and patients with AML with combined TP53/TET2 alterations displayed increased expression of the TIGIT ligand, CD155, on malignant cells. TIGIT-blocking antibodies augmented NK cell-mediated killing of Tp53/Tet2 double-mutant AML cells, reduced leukemic burden, and prolonged survival in Tp53/Tet2 double-KO mice. These findings describe a leukemia-promoting link between TET2 and TP53 mutations and highlight therapeutic strategies to overcome the immunosuppressive bone marrow environment in this adverse subtype of AML.
Keywords: Hematology; Inflammation; Leukemias; Mouse models; Oncology; p53.
Figures





References
-
- Loizou E, et al. A gain-of-function p53-mutant oncogene promotes cell fate plasticity and myeloid leukemia through the pluripotency factor FOXH1. Cancer Discov. 2019;9(7):962–979. doi: 10.1158/2159-8290.CD-18-1391. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

