Real-world effectiveness of ixazomib, lenalidomide and dexamethasone in Asians with relapsed/refractory multiple myeloma
- PMID: 40111640
- PMCID: PMC12014722
- DOI: 10.1007/s12185-025-03927-z
Real-world effectiveness of ixazomib, lenalidomide and dexamethasone in Asians with relapsed/refractory multiple myeloma
Abstract
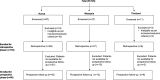
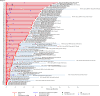
Randomized clinical trials have shown ixazomib, lenalidomide and dexamethasone (IRd) to be efficacious and safe in Asian patients with relapsed/refractory multiple myeloma (RRMM); however, real-world data are limited. The APEX study was a multicenter, observational cohort study of IRd conducted at 16 sites across South Korea, Malaysia, and Thailand. Overall, 104 patients treated with IRd during 2016-2023 were enrolled; data were collected by retrospective chart review and 6-month prospective follow-up. Median age at IRd initiation was 64.0 years. The primary endpoints of median time to next treatment (TTNT) and overall response rate (ORR) were 32.1 months and 72.1%, respectively (though ORR varied across countries). The secondary endpoint of median progression-free survival was 27.7 months, while median overall survival was not reached. Median TTNT and ORR were higher in elderly patients (≥65 and/or ≥70 years) than in the overall population. Adverse events occurred in 90.4% and serious adverse events occurred in 29.8% of all patients; common Grade ≥ 3 adverse drug reactions were pneumonia (9.6%), neutropenia (7.7%), and gastroenteritis (2.9%). This study demonstrated that IRd was safe and effective in real-world practice in Asia, including for elderly patients, and the results are aligned with TOURMALINE-MM1 and other real-world studies.
Keywords: Asia; Ixazomib; Real-world; Relapsed refractory multiple myeloma.
© 2025. Takeda Development Center Americas, Inc. (TDCA), Lexington, MA, USA.
Conflict of interest statement
Declarations. Conflicts of interest: Hye-won Heo was an employee of Takeda Pharmaceuticals Korea Co., Ltd. Ji Hyun Lee has received honoraria/research funding from Takeda, Amgen, Janssen, Celgene-BMS, Sanofi and JW Pharmaceutical, and was a consultant for Takeda, Amgen and Janssen. Youngil Koh holds a leadership position at Genome Opinion, holds stock/options in Genome Opinion, Curocell, Proteina and Tomocube, and has received research funding from Sanofi-Genzyme and Roche. Soo Chin Ng has received consultation fees/honorarium for participation in advisory boards from Roche, AstraZeneca, Novartis and BeiGene. The other authors have no conflicts of interest to declare.
Figures

References
-
- Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–34. - PubMed
-
- Lee JH, Kim SH, Kim HR, Min CK, Lee JJ, Shin HJ, et al. Real-world toxicity and effectiveness of ixazomib, lenalidomide, and dexamethasone in Korean patients with relapsed and/or refractory multiple myeloma. Int J Hematol. 2023;117(2):225–35. - PubMed
-
- Shirley M. Ixazomib: first global approval. Drugs. 2016;76(3):405–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

