Dosing and clinical outcomes of ruxolitinib in patients with myelofibrosis in a real-world setting: Interim results of the Italian observational study (ROMEI)
- PMID: 40111826
- PMCID: PMC11925231
- DOI: 10.1002/cncr.35801
Dosing and clinical outcomes of ruxolitinib in patients with myelofibrosis in a real-world setting: Interim results of the Italian observational study (ROMEI)
Abstract
Background: Myelofibrosis (MF) significantly impacts patients' overall survival (OS) and quality of life (QOL). This prospective study analyzed ruxolitinib dosing patterns and associated clinical outcomes in patients with MF over 12 months.
Methods: ROMEI, a multicenter, observational, ongoing study, enrolled 508 adult patients with MF treated with ruxolitinib. For the current interim analysis, eligible patients with baseline platelet values were categorized into two groups based on ruxolitinib starting dosage: as expected (AsEx, n = 174) and lower than expected (LtEx, n = 132); ruxolitinib dose changes, interruptions and time to permanent discontinuation were analyzed, along with symptoms response, health-related QOL scores, spleen response, OS, and safety.
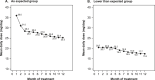
Results: Forty-three percent of patients started at a lower-than-expected dose. Both groups showed reduction in average daily ruxolitinib doses over 12 months. Symptoms response rate was similar in both groups at week 48 (40.8% AsEx vs 40.9% LtEx). The AsEx group demonstrated higher spleen response rates at both 24 weeks (50.0% vs 30.2%) and 48 weeks (57.7% vs 45.8%) with a shorter median time to first response (3.3 vs 11.1 months, p = .019) when compared to the LtEx group. Both groups showed upward trends in health-related QOL values. Estimated median OS was not reached for the AsEx group versus 4.7 years in the LtEx group (p = .014). Adverse events were reported in 87.4% and 84.9% of patients in the AsEx and LtEx groups, respectively.
Conclusions: The ROMEI study demonstrated the importance of optimal ruxolitinib dosage in patients with MF for maximum effectiveness and improved OS, with manageable safety.
Keywords: ROMEI study; dosing patterns; lower recommended dose; myelofibrosis; ruxolitinib; spleen response.
© 2025 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Massimo Breccia: received honoraria from Novartis, Pfizer, Incyte, BMS/Celgene, AbbVie, AOP, and GSK; Francesca Palandri: consultant and received honoraria from AbbVie, Amgen, AOP, BMS Celgene, Novartis, CTI, GSK, Grifols, Karyopharm, Morphosis, Sierra Oncology, and Sobi; Maurizio Martelli: no conflicts of interest; Francesco Mendicino: no conflicts of interest; Alessandra Malato: no conflicts of interest; Giuseppe A. Palumbo: honoraria for Advisory Boards/Meetings as speaker from Abbvie, AOP Orphan, AstraZeneca, Beigene, Bristol‐Myers Squibb, GSK, Incyte, Morphosys, and Novartis; support for attending meetings and travel from Abbvie, AOP Orphan, Beigene, Johnson & Johnson, Novartis, and Stemline Menarini; Silvia Sibilla: no conflicts of interest; Nicola Di Renzo: no conflicts of interest; Elisabetta Abruzzese: no conflicts of interest; Sergio Siragusa: no conflicts of interest; Monica Crugnola: no conflicts of interest; Carmine Selleri: no conflicts of interest; Fabrizio Pane: no conflicts of interest; Paolo Sportoletti: no conflicts of interest; Bruno Martino: no conflicts of interest; Stefana Impera: no conflicts of interest; Alessandra Ricco: no conflicts of interest; Maria Langella: no conflicts of interest; Paolo Ditonno: no conflicts of interest; Giuseppe Carli: no conflicts of interest; Federico Itri: no conflicts of interest; Anna Marina Liberati: no conflicts of interest; Tiziana Urbano: no conflicts of interest; Agostino Tafuri: no conflicts of interest; Vita Polizzi: no conflicts of interest; Domenico Pastore: no conflicts of interest; Erika Morsia: no conflicts of interest; Giulia Benevolo: no conflicts of interest; Giorgia Micucci: no conflicts of interest; Gabriella Farina: no conflicts of interest; Massimiliano Bonifacio: received honoraria from Novartis, Bristol Myers Squibb, Pfizer, Incyte, Ascentage Pharma, and Amgen; Elena Maria Elli: no conflicts of interest; Angelo Gardellini: no conflicts of interest; Valerio De Stefano: received honoraria from AbbVie, Bristol Myers Squibb, and Novartis; Giovanni Caocci: no conflicts of interest; Antonietta Pia Falcone: no conflicts of interest; Daniele Vallisa: no conflicts of interest; Marco Brociner: no conflicts of interest; Mario Tiribelli: received honoraria from Novartis, Bristol Myers Squibb, Incyte, Pfizer, and GSK; Gianni Binotto: honoraria from Novartis, Bristol Myers Squibb, Incyte, Pfizer, and AOP; Barbara Pocali: no conflicts of interest; Francesco Cavazzini: no conflicts of interest; Simona Tomassetti: no conflicts of interest; Francesca Lunghi: no conflicts of interest; Mauro Di Ianni: no conflicts of interest; Alessandro Allegra: no conflicts of interest; Barbara Anaclerio: no conflicts of interest; Serena Mazzotta: no conflicts of interest; Nicola Orofino: no conflicts of interest; Filippo Gherlinzoni: no conflicts of interest; Chiara Castiglioni: employee of Novartis Farma S.P.A.; Marina Landoni: employee of Novartis Farma S.P.A.; Diletta Valsecchi: employee of Novartis Farma S.P.A.; Michela Magnoli: employee of OPIS S.r.l.; Paola Guglielmelli: honoraria for lectures, presentations, speakers bureaus, manuscript writing, and educational events from Novartis, AbbVie, GSK, and AOP Orphan as well as participation on the advisory board of Novartis, Incyte, and GSK; Francesco Passamonti: honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, and educational events from Novartis, Bristol Myers Squibb, AbbVie, GSK, Janssen, and AOP Orphan as well as participation on the advisory board of Novartis, Bristol Myers Squibb/Celgene, GSK, AbbVie, AOP Orphan, Janssen, Karyiopharma, Kyowa Kirin and MEI, Sumitomo, and Kartos.
Figures