Gene deletion as a possible strategy adopted by New World Leishmania infantum to maximize geographic dispersion
- PMID: 40111992
- PMCID: PMC11975383
- DOI: 10.1371/journal.ppat.1012938
Gene deletion as a possible strategy adopted by New World Leishmania infantum to maximize geographic dispersion
Erratum in
-
Correction: Gene deletion as a possible strategy adopted by New World Leishmania infantum to maximize geographic dispersion.PLoS Pathog. 2025 Aug 28;21(8):e1013452. doi: 10.1371/journal.ppat.1013452. eCollection 2025 Aug. PLoS Pathog. 2025. PMID: 40875641 Free PMC article.
Abstract
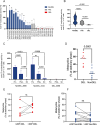
Background: The present study investigates implications of a sub-chromosomal deletion in Leishmania infantum strains, the causative agent of American Visceral Leishmaniasis (AVL). Primarily found in New World strains, the deletion leads to the absence of the ecto-3'-nucleotidase/nuclease enzyme, impacting parasite virulence, pathogenicity, and drug susceptibility. The factors favoring prevalence and the widespread geographic distribution of these deleted mutant parasites (DEL) in the NW (NW) are discussed under the generated data.
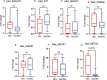
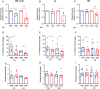
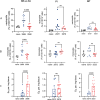
Methods: We conducted phenotypic assessments of the sub-chromosomal deletion through in vitro assays with axenic parasites and experimental infections in both in vitro and in vivo models of vertebrate and invertebrate hosts using geographically diverse mutant field isolates.
Results: Despite reduced pathogenicity, the DEL strains efficiently infect vertebrate hosts and exhibit relevant differences, including enhanced metacyclogenesis and colonization rates in sand flies, potentially facilitating transmission. This combination may represent a more effective way to maintain and disperse the transmission cycle of DEL strains.
Conclusions: Phenotypic assessments reveal altered parasite fitness, with potential enhanced transmissibility at the population level. Reduced susceptibility of DEL strains to miltefosine, a key drug in VL treatment, further complicates control efforts. The study underscores the importance of typing parasite genomes for surveillance and control, advocating for the sub-chromosomal deletion as a molecular marker in AVL management.
Copyright: © 2025 Florêncio et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Bussotti G, Li B, Pescher P, Vojtkova B, Louradour I, Pruzinova K, et al. Leishmania allelic selection during experimental sand fly infection correlates with mutational signatures of oxidative DNA damage. Proc Natl Acad Sci U S A. 2023;120(10):e2220828120. doi: 10.1073/pnas.2220828120 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

