Formation of perineuronal nets within a thalamocortical circuit shapes mechanical and thermal pain thresholds in mice with neuropathic pain
- PMID: 40112162
- PMCID: PMC12004981
- DOI: 10.1097/j.pain.0000000000003563
Formation of perineuronal nets within a thalamocortical circuit shapes mechanical and thermal pain thresholds in mice with neuropathic pain
Abstract
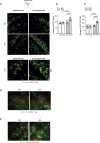
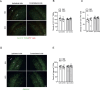
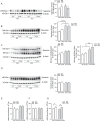
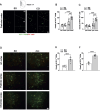
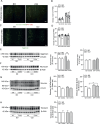
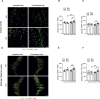
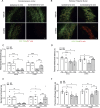
We moved from the hypothesis that perineuronal nets (PNNs), which are condensed structures of the extracellular matrix surrounding GABAergic interneurons in the forebrain, contribute to mechanisms of maladaptive neuronal plasticity underlying chronic pain. Here, we found that the density of PNNs labelled with the lectin Wisteria Floribunda Agglutinin (WFA) increased in the contralateral somatosensory cortex (SSC), medial prefrontal cortex (mPFC), reticular thalamic nucleus (RTN), and insular cortex of mice developing neuropathic pain in response to unilateral chronic constriction injury of the sciatic nerve. These regions are involved in neuronal circuits underlying perception, sufferance, embodiment, and top-down control of pain. At least in the SSC and mPFC, the increased density of WFA + PNNs was associated with an up-regulation of the proteoglycans, brevican and neurocan, as shown by immunoblot analysis. Enzymatic degradation of PNNs caused by local infusion of chondroitinase ABC in the contralateral SSC or RTN enhanced both mechanical and thermal pain thresholds in chronic constriction injury mice. In contrast, siRNA-induced knock-down of the PNN-degrading enzyme, type-9 matrix metalloproteinase (MMP-9), in the SSC or RTN lowered pain thresholds in sham-operated mice. These data, combined with our previous findings obtained in mice with chronic inflammatory pain, suggest that an enhanced formation/reduced degradation of WFA + PNNs in regions of the pain matrix is associated with different types of chronic pain and may drive mechanisms of nociceptive sensitization leading to reduced mechanical and thermal pain thresholds.
Keywords: Chondroitinase ABC; Neuropathic pain; Perineuronal nets; Prefrontal cortex; Reticular thalamic nuclei; Somatosensory cortex; Type-9 matrix metalloproteinase.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

