Macrophage HM13/SPP Enhances Foamy Macrophage Formation and Atherogenesis
- PMID: 40112173
- PMCID: PMC12079524
- DOI: 10.1002/advs.202412498
Macrophage HM13/SPP Enhances Foamy Macrophage Formation and Atherogenesis
Abstract
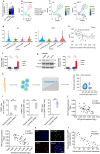
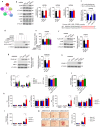
Aryl Hydrocarbon Receptor-Interacting Protein (AIP) reduces macrophage cholesterol-ester accumulation and may prevent atherogenic foamy macrophage formation. Analyzing AIP-associated regulatory gene networks can aid in identifying key regulatory mechanism(s) underlying foamy macrophage formation. A weighted gene co-expression network analysis on the Stockholm Atherosclerosis Gene Expression (STAGE) patient cohort identifies AIP as a negative correlate of Histocompatibility Minor 13 (HM13), which encodes the ER-associated degradation (ERAD) protein Signal Peptide Peptidase (HM13/SPP). The negative correlation between AIP and HM13/SPP on mRNA and protein levels is validated in oxLDL-stimulated macrophages and human plaque foamy macrophages. Mechanistically, AIP, via its chaperone interaction with Aryl Hydrocarbon Receptor (AHR), inhibits p38-c-JUN-mediated HM13 transactivation, thereby suppressing macrophage lipid accumulation. Myeloid HM13/SPP overexpression enhances oxLDL-induced foamy macrophage formation in vitro as well as atherogenesis and plaque foamy macrophage load in vivo, while myeloid HM13/SPP knockout produces the opposite effects. Mechanistically, myeloid HM13/SPP enhances oxLDL-induced foamy macrophage formation in vitro as well as atherogenesis and plaque foamy macrophage load in vivo via promoting ERAD-mediated proteasomal degradation of the metabolic regulator Heme Oxygenase-1 (HO-1). In conclusion, AIP downregulates macrophage HM13/SPP, a driver of oxLDL-induced lipid loading, foamy macrophage generation, and atherogenesis.
Keywords: AIP; HM13; HO‐1; SPP; atherosclerosis; foamy macrophage; macrophage.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
