A defining member of the new cysteine-cradle family is an aECM protein signalling skin damage in C. elegans
- PMID: 40112269
- PMCID: PMC11925461
- DOI: 10.1371/journal.pgen.1011593
A defining member of the new cysteine-cradle family is an aECM protein signalling skin damage in C. elegans
Abstract
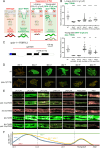
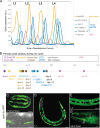
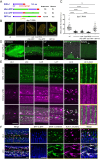
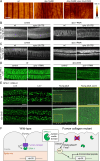
Apical extracellular matrices (aECMs) act as crucial barriers, and communicate with the epidermis to trigger protective responses following injury or infection. In Caenorhabditis elegans, the skin aECM, the cuticle, is produced by the epidermis and is decorated with periodic circumferential furrows. We previously showed that mutants lacking cuticle furrows exhibit persistent immune activation (PIA), providing a valuable model to study the link between cuticle damage and immune response. In a genetic suppressor screen, we identified spia-1 as a key gene downstream of furrow collagens and upstream of immune signalling. spia-1 expression oscillates during larval development, peaking between each moult together with patterning cuticular components. It encodes a secreted protein that localises to furrows. SPIA-1 shares a novel cysteine-cradle domain with other aECM proteins. SPIA-1 mediates immune activation in response to furrow loss and is proposed to act as an extracellular signal activator of cuticle damage. This research provides a molecular insight into intricate interplay between cuticle integrity and epidermal immune activation in C. elegans.
Copyright: © 2025 Sonntag et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Update of
-
A defining member of the new cysteine-cradle family is an aECM protein signalling skin damage in C. elegans.bioRxiv [Preprint]. 2024 Nov 5:2024.04.11.589058. doi: 10.1101/2024.04.11.589058. bioRxiv. 2024. Update in: PLoS Genet. 2025 Mar 20;21(3):e1011593. doi: 10.1371/journal.pgen.1011593. PMID: 39574764 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

