Molecular tumour board in gastrointestinal cancers
- PMID: 40112698
- PMCID: PMC11979463
- DOI: 10.1016/j.esmoop.2025.104510
Molecular tumour board in gastrointestinal cancers
Abstract
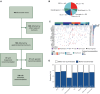
Background: Comprehensive genomic profiling (CGP) is being increasingly adopted in clinical practice to guide the use of molecularly guided treatment options (MGTOs). To optimize the integration of MGTOs in routine cancer care, molecular tumour boards (MTBs) have been established. Limited data are available to address the clinical value of implementing MTBs to inform treatment decision making in patients with gastrointestinal (GI) cancers.
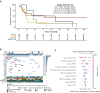
Materials and methods: We retrospectively retrieved medical records from patients with advanced GI cancers discussed at the European Institute of Oncology's MTB between August 2019 and December 2024. We evaluated clinical outcomes resulting from applying MGTOs in cancer care according to MTB recommendations, describing real-world progression-free (rwPFS) and overall survival (OS), and used the growth modulation index (GMI) (ratio of PFSMTB to PFSprior) to quantify the effectiveness of MTB's recommended cancer treatment in extending PFS.
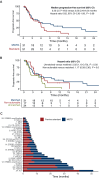
Results: Among 192 patients with GI cancers discussed at MTB, 139 (72.3%) received an MTB treatment recommendation. For patients with available follow-up data (n = 82), 31 patients (41.4%, 17.7% overall) received MGTOs, while 51 patients received standard treatments. Patients receiving MGTOs exhibited a longer rwPFS compared with cases receiving standard therapies [5.35 versus 3.55 months, hazard ratio (HR) 0.62, 95% confidence interval (CI) 0.36-1.08, P = 0.08] and with unmatched cases showing actionable biomarkers but not treated with targeted agents (n = 31) (rwPFS 5.35 versus 2.40 months, HR 0.49, 95% CI 0.27-0.90, P = 0.02). The use of MGTOs resulted in a GMI of 1.12 (interquartile range 0.68-2.36). The European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of molecular Targets (ESCAT) tier I-III treatments resulted in a restricted mean PFS gain of 4.87 months compared with standard therapies (95% CI 1.02-8.72 months, P = 0.01). No OS difference was observed between patients receiving MGTOs and standard treatments (P = 0.89).
Conclusions: Our results suggest that MTB-informed clinical decision making could provide valuable clinical benefits and expanded therapeutic options in patients affected by advanced GI cancers.
Keywords: gastrointestinal cancers; genomic profiling; molecular tumour board; next-generation sequencing; precision medicine; target therapy.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Prasad V., Fojo T., Brada M. Precision oncology: origins, optimism, and potential. Lancet Oncol. 2016;17(2):e81–e86. - PubMed
-
- Mosele M.F., Westphalen C.B., Stenzinger A., et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2024;35(7):588–606. - PubMed
-
- van de Haar J., Roepman P., Andre F., et al. ESMO Recommendations on clinical reporting of genomic test results for solid cancers. Ann Oncol. 2024;35(11):954–967. - PubMed
-
- Boscolo Bielo L., Trapani D., Curigliano G. Pharmacoeconomics of novel pharmacotherapies in triple-negative breast cancer. Expert Opin Pharmacother. 2023;24(7):789–801. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

