Modulation of ZnT-1 by Let7a unveils a therapeutic potential in amyotrophic lateral sclerosis
- PMID: 40113485
- PMCID: PMC12047506
- DOI: 10.1016/j.neurot.2025.e00571
Modulation of ZnT-1 by Let7a unveils a therapeutic potential in amyotrophic lateral sclerosis
Abstract
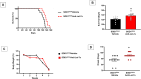
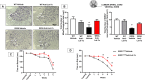
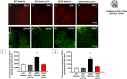
The imbalance in cellular ionic homeostasis represents a hallmark of several neurodegenerative diseases, including Amyotrophic Lateral Sclerosis (ALS). Zinc Transporter 1 (ZnT1), the first described member of the ZnT family, stands out as the sole member of the SLC30 family responsible for exporting cytosolic zinc to the extracellular space. While ZnT1 is expressed across all tissues and cell types studied, it exhibits the highest prominence within the central nervous system. In ALS SOD1G93A mice, a reduction in ZnT1 expression consistent with disease progression has been observed, prompting our investigation into its role in ALS pathophysiology. Remarkably, through the use of a sequence complementary to the microRNA let-7a (anti-Let-7a) able to modulate ZnT1 expression, we demonstrated in ALS mice its capability to: (1) prevent the reduction in ZnT1 levels in the spinal cord; (2) preserve motor neuron survival in the ventral spinal horn; (3) decrease astroglial and microglial activation while sparing resident microglial cells in the spinal cord; and (4) improve the lifespan and alleviate motor symptoms.
Keywords: ALS; G93A; ZnT1; microRNA.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Hand C.K., Rouleau G.A. Familial amyotrophic lateral sclerosis. Muscle Nerve. 2002;25(2):135–159. - PubMed
-
- Pasinelli P., Brown R.H. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7(9):710–723. - PubMed
-
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. - PubMed
-
- Barnham K.J., Bush A.I. Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Chem Biol. 2008;12(2):222–228. - PubMed
-
- Vonk W.I., Klomp L.W. Role of transition metals in the pathogenesis of amyotrophic lateral sclerosis. Biochem Soc Trans. 2008;36(Pt 6):1322–1328. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

