Deucravacitinib: Laboratory Parameters Across Phase 3 Plaque Psoriasis Trials
- PMID: 40113724
- PMCID: PMC11971090
- DOI: 10.1007/s13555-025-01362-w
Deucravacitinib: Laboratory Parameters Across Phase 3 Plaque Psoriasis Trials
Abstract
Introduction: Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, is approved in the USA and other countries for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy. In POETYK PSO-1 and PSO-2, deucravacitinib was superior to placebo and apremilast and well tolerated in patients with plaque psoriasis. Patients who completed PSO-1/PSO-2 could enroll in the POETYK long-term extension (LTE) trial. This analysis evaluates the effects of deucravacitinib on laboratory parameters.
Methods: POETYK PSO-1 and PSO-2 were 52-week, phase 3, double-blinded trials that randomized patients 1:2:1 to placebo, deucravacitinib 6 mg once daily, or apremilast 30 mg twice daily. At week 52, eligible patients enrolled in POETYK LTE and received open-label deucravacitinib. Mean changes from baseline in laboratory parameters, laboratory adverse events (AEs), and laboratory AEs resulting in discontinuation were evaluated over 3 years.
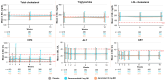
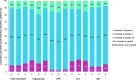
Results: A total of 1519 patients received one or more doses of deucravacitinib across trials. Total exposure over 3 years was 3294.3 person-years. No clinically relevant mean changes were observed in laboratory parameters. Grade ≥ 3 laboratory AEs were infrequent during the 1-year period, with incidence rates remaining stable in patients treated with deucravacitinib through 3 years. Most laboratory AEs remained at the same grade; shifts to higher grades were infrequent, with most increases being to grade ≤ 2. Discontinuations due to laboratory AEs were rare.
Conclusions: Deucravacitinib did not result in clinically meaningful changes in laboratory parameters over 3 years, including changes seen with Janus kinase (JAK) 1,2,3 inhibitors. Grade ≥ 3 laboratory AEs and discontinuations were rare.
Trial registration: ClinicalTrials.gov identifier, POETYK PSO-1 (NCT03624127), POETYK PSO-2 (NCT03611751), POETYK LTE (NCT04036435).
Keywords: Alanine aminotransferase; Aspartate aminotransferase; Creatine phosphokinase; Deucravacitinib; Liver function tests; Oral small molecule; Psoriasis; TYK2, tyrosine kinase 2; Triglycerides.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: April W. Armstrong has served as a research investigator, scientific advisor, and/or speaker for AbbVie, Almirall, Arcutis, Aslan Pharmaceuticals, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, EPI Health, Incyte, Janssen, Leo Pharma, Lilly, Mindera Health, Nimbus, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Leon Kircik has received research grants from AbbVie, Allergan, Almirall, Amgen, Arcutis, Boehringer Ingelheim, Breckinridge Pharma, Bristol Myers Squibb, Celgene, Cellceutix, Centocor, CombiMatrix, Connetics, Coria, Dermavant, Dermira, Dow Pharma, Dr. Reddy’s Laboratories, Galderma, Genentech, GSK, Idera, Johnson & Johnson, Leo Pharma, Lilly, Maruho, Merck, Medicis, Novartis AG, Pfizer, PharmaDerm, Promius, Stiefel, Sun Pharma, UCB, Valeant, and XenoPort, and has received honoraria from AbbVie, Allergan, Almirall, Amgen, Arcutis, Biogen Idec, Bristol Myers Squibb, Celgene, Cipher, Connetics, Dermavant, Dermira, Dr. Reddy’s Laboratories, Galderma, Genentech, GSK, Johnson & Johnson, Leo Pharma, Lilly, Merck, Novartis AG, PharmaDerm, Promius, Serono, Stiefel, Sun Pharma, Taro, UCB, and Valeant. Linda Stein Gold has served as a consultant, advisory board member, and/or speaker for AbbVie, Amgen, Arcutis, Aslan, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, Galderma, Incyte, Janssen, Leo Pharma, Lilly, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB. Bruce Strober has served as a consultant with honoraria for AbbVie, Acelyrin, Alamar, Almirall, Alumis, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Bristol Myers Squibb, Capital One, Celltrion, CorEvitas, Dermavant, Inmagene, Janssen/J&J Innovative Medicine, Kangpu Biopharmaceuticals, Leo Pharma, Lilly, Maruho, Meiji Seika Pharma, Monte Rosa Therapeutics, Novartis, Pfizer, Protagonist, RAPT Therapeutics, Regeneron, Sanofi, Sun Pharma, Takeda, TD Cowen, UCB, Union Therapeutics, Ventyx Biosciences, and vTv Therapeutics; as a speaker for AbbVie, Arcutis, Dermavant, Incyte, Janssen/J&J Innovative Medicine, Lilly, Regeneron, and Sanofi; as a co-scientific director (consulting fee) and investigator for the CorEvitas Psoriasis Registry; as editor-in-chief with an honorarium for the Journal of Psoriasis and Psoriatic Arthritis; and holds stock options in Connect Biopharma and Mindera Health. Claudia H. M. C. De Oliveira, John Vaile, Ying-Ming Jou, and Carolin Daamen are employees of and shareholders in Bristol Myers Squibb. Thomas Scharnitz was an employee of and shareholder in Bristol Myers Squibb at the time of study conduct and is now an employee of Novartis. Mark Lebwohl is an employee of Mount Sinai and receives research funds from AbbVie, Arcutis, Avotres, Boehringer Ingelheim, Cara Therapeutics, Clexio, Dermavant Sciences, Incyte, Inozyme, Janssen, Lilly, Pfizer, Sanofi-Regeneron, and UCB, and is a consultant for Almirall, AltruBio, Apogee, Arcutis, AstraZeneca, Atomwise, Avotres, Boehringer Ingelheim, Bristol Myers Squibb, Castle Biosciences, Celltrion, CorEvitas Psoriasis Registry, Dermavant, Dermsquared, Evommune, Facilitation of International Dermatology Education, Forte Biosciences, Galderma, Genentech, Incyte, Leo Pharma, Meiji Seika Pharma, Mindera Health, Pfizer, Sanofi-Regeneron, Seanergy, Strata, Takeda, Trevi, and Verrica. Mark Lebwohl is an Editorial Board member of Dermatology and Therapy. Mark Lebwohl was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Ethical Approval: These studies were conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines of the International Council for Harmonisation and in accordance with the European Union Directive 2001/20/EC and the US Code of Federal Regulations, Title 21, Part 50 (21CFR50), as well as applicable local requirements. The study protocols and amendments were approved by an institutional review board or independent ethics committee before initiation of the study at each site. All patients or their legal representatives provided written informed consent prior to study participation.
Figures

References
-
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11(502):eaaw1736. - PubMed
-
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62(20):8973–95. - PubMed
-
- Sotyktu [package insert]. Princeton, NJ, USA: Bristol Myers Squibb, 2022.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

