Engraftment outcome of patients with anti-HLA antibodies in HLA-mismatched peripheral blood stem cell transplantation
- PMID: 40113728
- PMCID: PMC12106477
- DOI: 10.1007/s12185-025-03952-y
Engraftment outcome of patients with anti-HLA antibodies in HLA-mismatched peripheral blood stem cell transplantation
Abstract
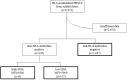
Anti-human leukocyte antigen (HLA) antibodies, particularly donor-specific HLA antibodies (DSA), negatively impact engraftment in hematopoietic cell transplantation. Past studies have proposed various interventions to reduce DSA, but these were primarily from single centers and not from large-scale registry data. Therefore, we conducted a retrospective analysis of nationwide registry data to examine the effects of anti-HLA antibodies on engraftment. Evaluable patients were classified into an anti-HLA antibody-negative group (n = 3657), an anti-HLA antibody-positive group (without high DSA) (n = 137), and a high-DSA (MFI > 5000) group (n = 8). Patient characteristics differed significantly between the anti-HLA antibody-negative and anti-HLA antibody-positive groups, and the number of patients with DSA was lower than expected. Statistical analyses revealed that the anti-HLA antibody-positive group had better neutrophil engraftment than the anti-HLA antibody-negative group (94.0% vs 84.2%, p < 0.001) but worse platelet engraftment (60.3% vs 64.9%, p = 0.047). In the high DSA group, two patients received a DSA-depleting intervention. Only one patient with an MFI of 5832 (without intervention) developed primary graft failure, while the remaining seven achieved engraftment. In this study, the effect of anti-HLA antibodies remained inconclusive, and the possibility of neutrophil engraftment with high-DSA levels was confirmed.
Keywords: Anti-HLA antibody; DSA; Engraftment; MFI; PBSCT.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Hirohisa Nakamae received research funding from Astellas Pharma Inc. and Novartis Pharma K.K., and honoraria from Astellas Pharma Inc., Kyowa Kirin Co., Ltd., NIPPON SHINYAKU Co., Ltd., and Novartis Pharma K.K. The authors declare no competing financial interests. One of the authors, Yoshinobu Kanda, is the editor of the International Journal of Hematology.
Figures

References
-
- Takanashi M, Atsuta Y, Fujiwara K, Kodo H, Kai S, Sato H, Kohsaki M, Azuma H, Tanaka H, Ogawa A, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839–46. - PubMed
-
- Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, Fujioka T, Tamaki H, Ikegame K, Okada M, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47(4):508–15. - PubMed
-
- Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, Chen H, Wang FR, Mo XD, Zhang YY, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015;8:84. - PMC - PubMed
-
- Bramanti S, Calafiore V, Longhi E, Mariotti J, Crespiatico L, Sarina B, De Philippis C, Nocco A, Santoro A, Castagna L. Donor-Specific Anti-HLA Antibodies in Haploidentical Stem Cell Transplantation with Post-Transplantation Cyclophosphamide: Risk of Graft Failure, Poor Graft Function, and Impact on Outcomes. Biol Blood Marrow Transplant. 2019;25(7):1395–406. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

