The BCL2 family: from apoptosis mechanisms to new advances in targeted therapy
- PMID: 40113751
- PMCID: PMC11926181
- DOI: 10.1038/s41392-025-02176-0
The BCL2 family: from apoptosis mechanisms to new advances in targeted therapy
Abstract
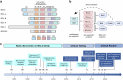
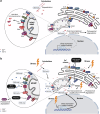
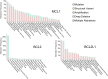
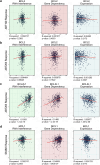
The B cell lymphoma 2 (BCL2) protein family critically controls apoptosis by regulating the release of cytochrome c from mitochondria. In this cutting-edge review, we summarize the basic biology regulating the BCL2 family including canonical and non-canonical functions, and highlight milestones from basic research to clinical applications in cancer and other pathophysiological conditions. We review laboratory and clinical development of BH3-mimetics as well as more recent approaches including proteolysis targeting chimeras (PROTACs), antibody-drug conjugates (ADCs) and tools targeting the BH4 domain of BCL2. The first BCL2-selective BH3-mimetic, venetoclax, showed remarkable efficacy with manageable toxicities and has transformed the treatment of several hematologic malignancies. Following its success, several chemically similar BCL2 inhibitors such as sonrotoclax and lisaftoclax are currently under clinical evaluation, alone and in combination. Genetic analysis highlights the importance of BCL-XL and MCL1 across different cancer types and the possible utility of BH3-mimetics targeting these proteins. However, the development of BH3-mimetics targeting BCL-XL or MCL1 has been more challenging, with on-target toxicities including thrombocytopenia for BCL-XL and cardiac toxicities for MCL1 inhibitors precluding clinical development. Tumor-specific BCL-XL or MCL1 inhibition may be achieved by novel targeting approaches using PROTACs or selective drug delivery strategies and would be transformational in many subtypes of malignancy. Taken together, we envision that the targeting of BCL2 proteins, while already a success story of translational research, may in the foreseeable future have broader clinical applicability and improve the treatment of multiple diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: MJSD has received research funding from LOXO and Beigene and financial assistance with meeting attendance from Beigene. GB is involved in a fee-for-service agreement with ReMynd. All other authors declare no conflict of interest.
Figures


References
-
- Newton, K., Strasser, A., Kayagaki, N. & Dixit, V. M. Cell death. Cell187, 235–256 (2024). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 70114124/Deutsche Krebshilfe (German Cancer Aid)
- DKS A2023_01/Deutsche Kinderkrebsstiftung (German Childhood Cancer Foundation)
- Junior Clinical Scientist programme/Goethe-Universität Frankfurt am Main (Goethe University Frankfurt am Main)
- PI20/00328/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- G0E7520N, G081821N and G094522N/Fonds Wetenschappelijk Onderzoek (Research Foundation Flanders)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

