Regulatory T cells in the mouse hypothalamus control immune activation and ameliorate metabolic impairments in high-calorie environments
- PMID: 40113758
- PMCID: PMC11926360
- DOI: 10.1038/s41467-025-57918-z
Regulatory T cells in the mouse hypothalamus control immune activation and ameliorate metabolic impairments in high-calorie environments
Abstract
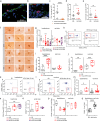
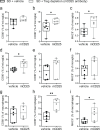
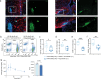
The hypothalamus in the central nervous system (CNS) has important functions in controlling systemic metabolism. A calorie-rich diet triggers CNS immune activation, impairing metabolic control and promoting obesity and Type 2 Diabetes (T2D), but the mechanisms driving hypothalamic immune activation remain unclear. Here we identify regulatory T cells (Tregs) as key modulators of hypothalamic immune responses. In mice, calorie-rich environments activate hypothalamic CD4+ T cells, infiltrating macrophages and microglia while reducing hypothalamic Tregs. mRNA profiling of hypothalamic CD4+ T cells reveals a Th1-like activation state, with increased Tbx21, Cxcr3 and Cd226 but decreased Ccr7 and S1pr1. Importantly, results from Treg loss-of function and gain-of-function experiments show that Tregs limit hypothalamic immune activation and reverse metabolic impairments induced by hyper-caloric feeding. Our findings thus help refine the current model of Treg-centered immune-metabolic crosstalk in the brain and may contribute to the development of precision immune modulation for obesity and diabetes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: As a scientist, M.H.T. participated in a scientific advisory board meeting of ERX Pharmaceuticals, Inc., Cambridge, MA, in 2019. He was a member of the Research Cluster Advisory Panel (ReCAP) of the Novo Nordisk Foundation between 2017 and 2019. He received funding for his research projects by Novo Nordisk (2016–2020) and Sanofi-Aventis (2012–2019). He consulted twice for Böhringer Ingelheim Pharma GmbH & Co. KG (2020 & 2021) and delivered a scientific lecture for Sanofi-Aventis Deutschland GmbH (2020) and for AstraZeneca GmbH (2024). As CEO and CSO of Helmholtz Munich, he is co-responsible for countless collaborations of the employees with a multitude of companies and institutions, worldwide. In this capacity, he discusses potential projects with and has signed/signs contracts for the centers institute(s) related to research collaborations worldwide, including but not limited to pharmaceutical corporations like Boehringer Ingelheim, Novo Nordisk, Roche Diagnostics, Arbormed, Eli Lilly, SCG Cell Therapy and others. As the CEO of Helmholtz Munich, he was/is further overall responsible for commercial technology transfer activities. M.H.T. confirms that to the best of his knowledge, none of the above funding sources or collaborations were involved in or had an influence on the preparation of this manuscript. The remaining authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
- Excellence Program for Outstanding Female Scientists/Helmholtz Association
- Initiative and Networking Fund/Helmholtz Association
- EFSD/JDRF/Lilly Programme on Type 1 Diabetes Research 2020/European Foundation for the Study of Diabetes (EFSD)
- Project-ID 210592381 - SFB 1054 (B11)/Deutsche Forschungsgemeinschaft (German Research Foundation)
- project number 490846870 - TRR355/1 TPA02/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 490846870 - TRR355/1 TPB02/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 490846870 - TRR355/1 TPA09/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 490846870 - TRR355/1 TPB05/Deutsche Forschungsgemeinschaft (German Research Foundation)
- DFG-CRC1811 (B02)/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

