Structure and unusual binding mechanism of the hyaluronan receptor LYVE-1 mediating leucocyte entry to lymphatics
- PMID: 40113779
- PMCID: PMC11926218
- DOI: 10.1038/s41467-025-57866-8
Structure and unusual binding mechanism of the hyaluronan receptor LYVE-1 mediating leucocyte entry to lymphatics
Abstract
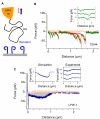
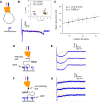
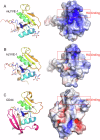
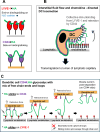
Immune surveillance involves the continual migration of antigen-scavenging immune cells from the tissues to downstream lymph nodes via lymphatic vessels. To enable such passage, cells first dock with the lymphatic entry receptor LYVE-1 on the outer surface of endothelium, using their endogenous hyaluronan glycocalyx, anchored by a second hyaluronan receptor, CD44. Why the process should require two different hyaluronan receptors and by which specific mechanism the LYVE-1•hyaluronan interaction enables lymphatic entry is however unknown. Here we describe the crystal structures and binding mechanics of murine and human LYVE-1•hyaluronan complexes. These reveal a highly unusual, sliding mode of ligand interaction, quite unlike the conventional sticking mode of CD44, in which the receptor grabs free hyaluronan chain-ends and winds them in through conformational re-arrangements in a deep binding cleft, lubricated by a layer of structured waters. Our findings explain the mode of action of a dedicated lymphatic entry receptor and define a distinct, low tack adhesive interaction that enables migrating immune cells to slide through endothelial junctions with minimal resistance, while clinging onto their hyaluronan glycocalyx for essential downstream functions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- MC_UU_00008/2/RCUK | Medical Research Council (MRC)
- MR/N000331/1/RCUK | Medical Research Council (MRC)
- MR/X013227/1/RCUK | Medical Research Council (MRC)
- BB/X007278/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/R000174/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/X00158X/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- 795605/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- ERC-2012-StG-306435/EC | EC Seventh Framework Programm | FP7 Ideas: European Research Council (FP7-IDEAS-ERC - Specific Programme: "Ideas" Implementing the Seventh Framework Programme of the European Community for Research, Technological Development and Demonstration Activities (2007 to 2013))
LinkOut - more resources
Full Text Sources
Miscellaneous

