Structural recognition and stabilization of tyrosine hydroxylase by the J-domain protein DNAJC12
- PMID: 40113792
- PMCID: PMC11926245
- DOI: 10.1038/s41467-025-57733-6
Structural recognition and stabilization of tyrosine hydroxylase by the J-domain protein DNAJC12
Abstract
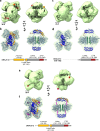
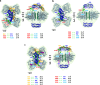
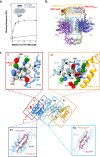
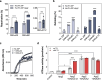
Pathogenic variants of the J-domain protein DNAJC12 cause parkinsonism, which is associated with a defective interaction of DNAJC12 with tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine biosynthesis. In this work, we characterize the formation of the TH:DNAJC12 complex, showing that DNAJC12 binding stabilizes both TH and the variant TH-p.R202H, associated with TH deficiency. This binding delays their time-dependent aggregation in an Hsp70-independent manner, while preserving TH activity and feedback regulatory inhibition by dopamine. DNAJC12 alone barely activates Hsc70 but synergistically stimulates Hsc70 ATPase activity when complexed with TH. Cryo-electron microscopy supported by crosslinking-mass spectroscopy reveals two DNAJC12 monomers bound per TH tetramer, each embracing one of the two regulatory domain dimers, leaving the active sites available for substrate, cofactor and inhibitory dopamine interaction. Our results also reveal the key role of the C-terminal region of DNAJC12 in TH binding, explaining the pathogenic mechanism of the DNAJC12 disease variant p.W175Ter.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Balchin, D., Hayer-Hartl, M. & Hartl, F. U. In vivo aspects of protein folding and quality control. Science353, aac4354 (2016). - PubMed
-
- Wittung-Stafshede, P., Guidry, J., Horne, B. E. & Landry, S. J. The J-domain of Hsp40 couples ATP hydrolysis to substrate capture in Hsp70. Biochemistry42, 4937–4944 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

