Cas12a-knock-in mice for multiplexed genome editing, disease modelling and immune-cell engineering
- PMID: 40114032
- PMCID: PMC12360953
- DOI: 10.1038/s41551-025-01371-2
Cas12a-knock-in mice for multiplexed genome editing, disease modelling and immune-cell engineering
Abstract
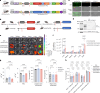
The pleiotropic effects of human disease and the complex nature of gene-interaction networks require knock-in mice allowing for multiplexed gene perturbations. Here we describe a series of knock-in mice with a C57BL/6 background and with the conditional or constitutive expression of LbCas12a or of high-fidelity enhanced AsCas12a, which were inserted at the Rosa26 locus. The constitutive expression of Cas12a in the mice did not lead to discernible pathology and enabled efficient multiplexed genome engineering. We used the mice for the retrovirus-based immune-cell engineering of CD4+ and CD8+ T cells, B cells and bone-marrow-derived dendritic cells, for autochthonous cancer modelling through the delivery of multiple CRISPR RNAs as a single array using adeno-associated viruses, and for the targeted genome editing of liver tissue using lipid nanoparticles. We also describe a system for simultaneous dual-gene activation and knockout (DAKO). The Cas12a-knock-in mice and the viral and non-viral delivery vehicles provide a versatile toolkit for ex vivo and in vivo applications in genome editing, disease modelling and immune-cell engineering, and for the deconvolution of complex gene interactions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.C. is a (co)founder of EvolveImmune Tx, Cellinfinity Bio, MagicTime Med and Chen Consulting, unrelated to this study. The other authors declare no competing interests.
Figures




References
-
- Wang, J. Y. & Doudna, J. A. CRISPR technology: a decade of genome editing is only the beginning. Science379, eadd8643 (2023). - PubMed
-
- Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol.38, 824–844 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- RF1DA048811/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- K99CA282989/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- F30CA250249/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- RF1 DA048811/DA/NIDA NIH HHS/United States
- R01 CA231112/CA/NCI NIH HHS/United States
- DP2 CA238295/CA/NCI NIH HHS/United States
- T32 GM007205/GM/NIGMS NIH HHS/United States
- F30 CA250249/CA/NCI NIH HHS/United States
- U54CA209992/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- DP2CA238295/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- K99 CA282989/CA/NCI NIH HHS/United States
- U54 CA209992/CA/NCI NIH HHS/United States
- R33 CA281702/CA/NCI NIH HHS/United States
- T32GM007205/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R33CA281702/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R33 CA225498/CA/NCI NIH HHS/United States
- R01CA231112/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

