Assessing the consistency of FIB-4, APRI, and GPR in evaluating significant liver fibrosis and cirrhosis in COVID-19 patients with concurrent liver diseases
- PMID: 40114058
- PMCID: PMC11927168
- DOI: 10.1186/s12876-025-03770-w
Assessing the consistency of FIB-4, APRI, and GPR in evaluating significant liver fibrosis and cirrhosis in COVID-19 patients with concurrent liver diseases
Abstract
Objective: This study investigated the consistency of the FIB-4, APRI, and GPR indices in assessing significant liver fibrosis and cirrhosis in patients with Coronavirus Disease 2019(COVID-19) who also suffer from various liver diseases, providing references for the clinical selection and application for non-invasive assessment methods.
Methods: The study evaluated 744 COVID-19 patients with coexisting liver diseases: 508 cases with non-alcoholic fatty liver disease (NAFLD), 158 cases with chronic hepatitis B (CHB), and 78 cases with a combination of both ailments. FIB-4, APRI, and GPR were employed to assess significant liver fibrosis and cirrhosis. Concordance among the methods was determined using Kappa analysis, and receiver operating characteristic (ROC) curves helped identify the optimal cutoff values for each index.
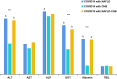
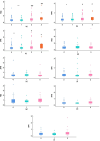
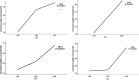
Results: For COVID-19 patients with NAFLD, Kappa values for significant liver fibrosis were 0.81, 0.90, 0.80, and 0.79, and for cirrhosis, they were 0.88, 0.97,0.88, and 0.88, respectively (all p < 0.05). Among those with CHB, Kappa values were 0.81, 0.81, 0.83, and 0.75 for fibrosis, and0.87, 0.91, 0.88, and 0.92 for cirrhosis (all p < 0.05). In patients with coexisting liver diseases, the values were 0.87, 0.86, 0.86, and 0.78 for fibrosis, and 0.67, 0.69, 0.54, and 0.81for cirrhosis (all p < 0.05). Linear trend analysis revealed significant relationships between FIB-4 values, APRI values, GPR values, and the severity of COVID-19 (χ2 trend: 15.205,35.114, and 13.973, respectively, all p < 0.001), between FIB-4 values and APRI values and the coronavirus negative conversion time (all p < 0.05) in COVID-19 with NAFLD, and between FIB-4 values and GPR values and the coronavirus negative conversion time in patients with COVID-19 with CHB(all p < 0.05).
Conclusion: Using the current cutoff values, the non-invasive assessments demonstrated almost perfect consistency in evaluating significant liver fibrosis and cirrhosis in COVID-19 patients with liver diseases, though FIB-4 and GPR showed moderate consistency in cirrhosis evaluation in patients with coexisting liver conditions. Moreover, it also indicated that increased liver fibrosis correlates with more severe COVID-19 and prolonged coronavirus negative conversion time.
Keywords: Chronic hepatitis B(CHB); Cirrhosis; Coronavirus disease 2019(COVID-19); Kappa value; Liver fibrosis; Non-alcoholic fatty liver disease(NAFLD); Non-invasive assessment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki ( https://www.wma.net/policies-post/wma-declaration-of- helsinki/). Ethical approval for this research was obtained from the Ethics Committee of the Public Health and Clinical Center of Chengdu (No.: PJ-K2020-26-01). The ethics committee/institutional review board waived the requirement for written informed consent from the participants or their legal guardians/next of kin due to the retrospective nature of the study, the use of anonymized data, and the provision of comprehensive verbal and written information to all patients regarding the study’s purpose and potential implications. The study was conducted in accordance with local legislation and institutional requirements. Consent for publication: All of the participants understand that the information will be published without their child or ward’s/their relative’s (circle as appropriate) name attached but that full anonymity cannot be guaranteed. All of the participants understand that the text and any pictures or videos published in the article will be freely available on the internet and may be seen by the general public. The pictures, videos and text may also appear on other websites or in print and may be translated into other languages or used for commercial purposes. All of the participants were offered the opportunity to read the manuscript. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
The gamma-glutamyl transpeptidase to platelet ratio for non-invasive assessment of liver fibrosis in patients with chronic hepatitis B and non-alcoholic fatty liver disease.Oncotarget. 2017 Apr 25;8(17):28641-28649. doi: 10.18632/oncotarget.16162. Oncotarget. 2017. PMID: 28415736 Free PMC article.
-
Gamma-Glutamyl Transpeptidase-to-Platelet ratio predicts liver fibrosis in patients with concomitant chronic hepatitis B and nonalcoholic fatty liver disease.J Clin Lab Anal. 2022 Aug;36(8):e24596. doi: 10.1002/jcla.24596. Epub 2022 Jul 9. J Clin Lab Anal. 2022. PMID: 35808928 Free PMC article.
-
Value of gamma-glutamyltranspeptidase-to-platelet ratio in diagnosis of hepatic fibrosis in patients with chronic hepatitis B.World J Gastroenterol. 2017 Nov 7;23(41):7425-7432. doi: 10.3748/wjg.v23.i41.7425. World J Gastroenterol. 2017. PMID: 29151696 Free PMC article.
-
Aspartate aminotransferase to platelet ratio can reduce the need for transient elastography in Chinese patients with chronic hepatitis B.Medicine (Baltimore). 2019 Dec;98(49):e18038. doi: 10.1097/MD.0000000000018038. Medicine (Baltimore). 2019. PMID: 31804310 Free PMC article.
-
The Gamma-Glutamyl-Transpeptidase to Platelet Ratio Does not Show Advantages than APRI and Fib-4 in Diagnosing Significant Fibrosis and Cirrhosis in Patients With Chronic Hepatitis B: A Retrospective Cohort Study in China.Medicine (Baltimore). 2016 Apr;95(16):e3372. doi: 10.1097/MD.0000000000003372. Medicine (Baltimore). 2016. PMID: 27100421 Free PMC article.
References
-
- Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses andcytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

