Metabolomic profiling reveals early biomarkers of gestational diabetes mellitus and associated hepatic steatosis
- PMID: 40114104
- PMCID: PMC11927189
- DOI: 10.1186/s12933-025-02645-4
Metabolomic profiling reveals early biomarkers of gestational diabetes mellitus and associated hepatic steatosis
Abstract
Background: This study aims to identify early metabolomic biomarkers of gestational diabetes mellitus (GDM) and evaluate their association with hepatic steatosis.
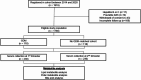
Methods: We compared maternal serum metabolomic profiles between women who developed GDM (n = 118) and matched controls (n = 118) during the first (10-14 gestational weeks) and second (24-28 gestational weeks) trimesters using ultra-performance liquid chromatography coupled with mass spectrometry. Mediation analysis was performed to evaluate the mediating role of metabolic dysfunction-associated steatotic liver disease (MASLD) in the relationship between metabolites and subsequent development of GDM. A refined prediction model was developed to predict GDM using established clinical factors and selected metabolites.
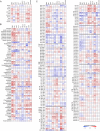
Results: Significant alterations in circulating metabolites, including amino acids, bile acids, and phospholipids, were observed in the GDM group compared to controls during early pregnancy. Mediation analysis revealed that several metabolites, including glycocholic acid (proportion mediated (PM) = 31.9%), butanoyl carnitine (PM = 25.7%), and uric acid (PM = 22.4%), had significant indirect effects on GDM incidence mediated by hepatic steatosis. The refined prediction model composed of clinical factors and selected metabolites in the first trimester demonstrated higher performance in predicting GDM development than the established prediction model composed solely of clinical factors (AUC, 0.85 vs. 0.63, p < 0.001).
Conclusions: Women who developed GDM exhibited altered metabolomic profiles from early pregnancy, which showed a significant correlation with GDM, with MASLD as a mediator. Selected metabolomic biomarkers may serve as predictive markers and potential targets for early risk assessment and intervention in GDM.
Research insights: WHAT IS CURRENTLY KNOWN ABOUT THIS TOPIC?: Gestational diabetes mellitus (GDM) is a common pregnancy complication with significant health risks. Early identification of women at high risk for GDM is crucial for timely intervention and improved outcomes. WHAT IS THE KEY RESEARCH QUESTION?: What alterations in circulating metabolites during early pregnancy are associated with subsequent GDM development? Does metabolic dysfunction-associated steatotic liver disease (MASLD) mediate the association between specific metabolites and GDM risk? WHAT IS NEW?: Significant alterations in bile acids, amino acids, phosphatidylethanolamines, and phosphatidylinositols were observed in early pregnancy sera of women who later developed GDM. MASLD significantly mediated the effects of several metabolites on GDM risk, with mediation proportions ranging from 9.7 to 31.9%. A refined prediction model composed of clinical factors and metabolites significantly improved the performance in predicting GDM development. HOW MIGHT THIS STUDY INFLUENCE CLINICAL PRACTICE?: These results provide new insights into early metabolic alterations associated with GDM development and highlight the potential mediating role of MASLD. This comprehensive metabolomic approach may contribute to the development of improved risk prediction models and targeted interventions for GDM prevention.
Keywords: Early biomarker; Gestational diabetes mellitus; Hepatic steatosis; Mediation analysis; Metabolomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
-
- Sweeting A, Hannah W, Backman H, Catalano P, Feghali M, Herman WH, Hivert MF, Immanuel J, Meek C, Oppermann ML, et al. Epidemiology and management of gestational diabetes. Lancet. 2024;404(10448):175–92. - PubMed
-
- Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

