Selenium nanoparticles activate selenoproteins to mitigate septic lung injury through miR-20b-mediated RORγt/STAT3/Th17 axis inhibition and enhanced mitochondrial transfer in BMSCs
- PMID: 40114196
- PMCID: PMC11924768
- DOI: 10.1186/s12951-025-03312-2
Selenium nanoparticles activate selenoproteins to mitigate septic lung injury through miR-20b-mediated RORγt/STAT3/Th17 axis inhibition and enhanced mitochondrial transfer in BMSCs
Abstract
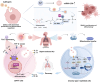
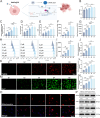
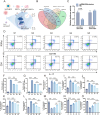
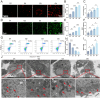
Sepsis-induced acute lung injury (ALI) remains a critical clinical challenge with complex inflammatory pathogenesis. While bone marrow mesenchymal stem cells (BMSCs) demonstrate therapeutic potential through anti-inflammatory and cytoprotective effects, their age-related functional decline limits clinical utility. This study developed chitosan-functionalized selenium nanoparticles (SeNPs@CS, 100 nm) to rejuvenate BMSCs through miR-20b-mediated selenoprotein biosynthesis. Mechanistic investigations revealed that SeNPs@CS-treated BMSCs exhibited enhanced mitochondrial transfer capacity, delivering functional mitochondria to damaged alveolar epithelial cells (AECII) for cellular repair. Concurrently, miR-20b upregulation suppressed the RORγt/STAT3/Th17 axis, reducing pro-inflammatory Th17 cell differentiation in CD4+ T lymphocytes. The dual-target mechanism integrates immunomodulation via Th17 pathway inhibition with mitochondrial rejuvenation therapy, representing a paradigm-shifting approach for ALI management. These engineered BMSCs mitigated inflammatory markers in murine models, demonstrating superior efficacy to conventional BMSC therapies. Our findings establish SeNPs@CS-modified BMSCs as a novel therapeutic platform combining nanotechnology-enhanced stem cell engineering with precision immunometabolic regulation, providing new avenues for the treatment of sepsis-induced ALI.
Keywords: Acute lung injury; BMSCs; Mitochondrial transfer; RORγt/STAT3; Selenium; Th17.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experimental care and operating procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals and approved by by the the Animal Ethical and Welfare Committee (Approval number: IACUC-MIS2023075). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Ericson JE, Agthe AG, Weitkamp JH. Late-Onset sepsis: epidemiology, microbiology, and controversies in practice. Clin Perinatol. 2025;52(1):33–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

