A reciprocal relationship between markers of genomic DNA damage and alpha-synuclein pathology in dementia with Lewy bodies
- PMID: 40114198
- PMCID: PMC11927131
- DOI: 10.1186/s13024-025-00813-4
A reciprocal relationship between markers of genomic DNA damage and alpha-synuclein pathology in dementia with Lewy bodies
Abstract
Background: DNA damage and DNA damage repair (DDR) dysfunction are insults with broad implications for cellular physiology and have been implicated in various neurodegenerative diseases. Alpha-synuclein (aSyn), a pre-synaptic and nuclear protein associated with neurodegenerative disorders known as synucleinopathies, has been associated with DNA double strand break (DSB) repair. However, although nuclear aSyn pathology has been observed in cortical tissue of dementia with Lewy body (DLB) cases, whether such nuclear pathology coincides with the occurrence of DNA damage has not previously been investigated. Moreover, the specific types of DNA damage elevated in DLB cases and the contribution of DNA damage towards Lewy body (LB) formation is unknown.
Methods: DNA damage and aSyn pathology were assessed in fixed lateral temporal cortex from clinically and neuropathologically confirmed DLB cases and controls, as well as in cortical tissue from young 3-month-old presymptomatic A30P-aSyn mice. Frozen lateral temporal cortex from DLB and control cases was subject to nuclear isolation, western blotting, aSyn seed amplification and proteomic characterisation via mass spectrometry.
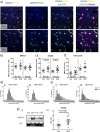
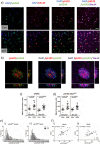
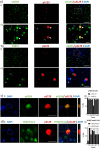
Results: We detected seed-competent nuclear aSyn, and elevated nuclear serine-129 phosphorylation in DLB temporal cortex, alongside the accumulation of DSBs in neuronal and non-neuronal cellular populations. DNA damage was also present in cortical tissue from presymptomatic A30P mice, demonstrating it is an early insult closely associated with pathogenic aSyn. Strikingly, in postmortem DLB tissue, markers of genomic DNA damage-derived cytoplasmic DNA (CytoDNA) were evident within the majority of LBs examined. The observed cellular pathology was consistent with nuclear upregulation of associated DDR proteins, particularly those involved in base excision repair and DSB repair pathways.
Conclusions: Collectively our study demonstrates the accumulation of seed-competent pathological nuclear associated aSyn, alongside nuclear DNA damage and the potential involvement of DNA damage derived cytoDNA species in cytoplasmic aSyn pathology. Ultimately, our study supports the hypothesis of a reciprocal relationship between aSyn pathology and nuclear DNA damage and highlights a potential underlying role for DNA damage in pathological mechanisms relevant to DLB, as well as other synucleinopathies, opening novel possibilities for diagnosis and treatment.
Keywords: Alpha-synuclein; DNA damage; synucleinopathy; Parkinson’s disease; Dementia with Lewy bodies.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The use of human tissue throughout this study was in accordance with Newcastle University Ethics Board (The Joint Ethics Committee of Newcastle and North Tyneside Health Authority, reference: 08/H0906/136). Mice from which tissue was obtained were housed and subject to procedures in accordance with the UK Animals (Scientific Procedures) Act 1986 and European Union directive 2010/63EU. Consent for publication: N/A. Competing interests: N/A.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

