Antibody-drug conjugates in breast cancer: current evidence and future directions
- PMID: 40114224
- PMCID: PMC11924693
- DOI: 10.1186/s40164-025-00632-9
Antibody-drug conjugates in breast cancer: current evidence and future directions
Abstract
Antibody-drug conjugates (ADCs) are a rapidly evolving class of antitumor drugs and have already revolutionized the treatment strategy of many hematologic and solid cancers. So far, trastuzumab emtansine (T-DM1), trastuzumab deruxtecan (T-DXd), sacituzumab govitecan (SG) and datopotamab deruxtecan (Dato-DXd) are the four ADCs that have been approved by US food and drug administration (FDA) in treatment of breast cancer, and SKB264 has been approved by Chinese national medical products administration (NMPA). Many ADCs for treatment of breast cancer are currently being tested in late-phase clinical trials, with several encouraging results achieved recently. However, major issues arise during the use of ADCs, including emergence of acquired resistance, occurrence of treated-related toxicities, and identification of biomarkers of response and resistance. ADCs are being increasingly tested in combination with other agents, and novel next-generation ADC development is progressing rapidly. A better understanding of the design and development of ADCs will promote ADC development for cancer treatment. In this review, we aim to provide a broad overview of the design and the recent advances of ADCs in breast cancer. We also propose several notable future directions of ADCs in treatment of breast cancer.
Keywords: Antibody-drug conjugate; Biomarker; Breast cancer; Combination therapy; Trials.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors have agreed to publish the present work in its submitted form. Competing interests: The authors declare no competing interests.
Figures
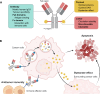
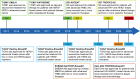
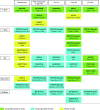

References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. - PubMed
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. - PubMed
-
- Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–69. - PubMed
-
- Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov. 2023;22(8):641–61. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

