Activin-A urine levels correlate with radiological patterns in preterm infants complicated by intraventricular hemorrhage
- PMID: 40114243
- PMCID: PMC11927341
- DOI: 10.1186/s13052-025-01938-4
Activin-A urine levels correlate with radiological patterns in preterm infants complicated by intraventricular hemorrhage
Abstract
Background: To validate the role of Activin A in the early diagnosis and prognosis of preterm newborns at risk for intraventricular hemorrhage and neurological sequelae by means of cerebral ultrasound and magnetic resonance imaging (MRI), currently considered standard of care procedures.
Methods: We conducted an observational case-control study in 46 preterm newborns, 23 with intraventricular hemorrhage (IVH group) and 23 controls matched for gestational age. Standard clinical, laboratory, cerebral ultrasound monitoring procedures and Activin A urine measurement were performed at four time-points (first void, 24, 48, 96 h) after birth. Cerebral MRI was performed at 40-42 weeks of corrected gestational age.
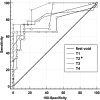
Results: Elevated (P < 0.001, for all) Activin A levels were observed in the IVH group at all monitoring time-point. Activin A correlated (P < 0.05, for all) with intraventricular hemorrhage grade on cerebral ultrasound. At the cut-off of 0.08 pg/mL Activin A at 48-h achieved the best sensitivity, specificity, positive/negative predictive values as early predictor of an abnormal MRI pattern (area under the curve: 0.93).
Conclusions: The present data showing a correlation among Activin A, cerebral ultrasound and MRI provide further support to Activin A inclusion in clinical daily management of cases at risk for intraventricular hemorrhage and adverse neurological outcome.
Keywords: Activin A; Cerebral ultrasound; Intraventricular hemorrhage; Magnetic resonance imaging; Preterm infants.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013). The Local Ethic Committees of the Cooperative Multitask against Brain Injury of Neonates (CoMBINe) International Network approved the study protocol. Consent for publication: Informed consent was obtained from all individuals included in this study. Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- M.o. Dimes, PMNCH, S.t. Children, WHO, in: Born too soon: the global action report on preterm birth. Howson C, Kinney M, Lawn J, Eds. (World Health Organization, Geneva, Switzerland, 2012.
-
- Leijser LM, de Vries LS. Preterm brain injury: germinal matrix–intraventricular hemorrhage and post-hemorrhagic ventricular dilatation. Hand Clin Neurol. 2019;162:173–99. - PubMed
-
- Serpero LD, Bellissima V, Colivicchi M, Sabatini M, Frigiola A, Ricotti A, et al. Next generation biomarkers for brain injury. J Matern Fetal Neonatal Med. 2013;26:44–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

