Microvascular aberrations found in human polycystic kidneys are an early feature in a Pkd1 mutant mouse model
- PMID: 40114603
- PMCID: PMC12067086
- DOI: 10.1242/dmm.052024
Microvascular aberrations found in human polycystic kidneys are an early feature in a Pkd1 mutant mouse model
Abstract
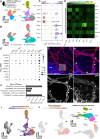
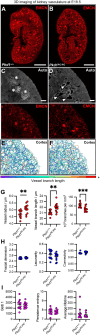
Therapies targeting blood vessels hold promise for autosomal dominant polycystic kidney disease (ADPKD), the most common inherited disorder causing kidney failure. However, the onset and nature of kidney vascular abnormalities in ADPKD are poorly defined. Accordingly, we employed a combination of single-cell transcriptomics; three-dimensional imaging with geometric, topological and fractal analyses; and multimodal magnetic resonance imaging with arterial spin labelling to investigate aberrant microvasculature in ADPKD kidneys. Within human ADPKD kidneys with advanced cystic pathology and excretory failure, we identified a molecularly distinct blood microvascular subpopulation, characterised by impaired angiogenic signalling and metabolic dysfunction, differing from endothelial injury profiles observed in non-cystic human kidney diseases. Next, Pkd1 mutant mouse kidneys were examined postnatally, when cystic pathology is well established, but before excretory failure. An aberrant endothelial subpopulation was also detected, concurrent with reduced cortical blood perfusion. Disorganised kidney cortical microvasculature was also present in Pkd1 mutant mouse fetal kidneys when tubular dilation begins. Thus, aberrant features of cystic kidney vasculature are harmonised between human and mouse ADPKD, supporting early targeting of the vasculature as a strategy to ameliorate ADPKD progression.
Keywords: Magnetic resonance imaging; Nephrology; Perfusion; Single-cell RNA sequencing; Three-dimensional microscopy; Vasculature.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




References
-
- Agterberg, F. P. (2013). Fractals and spatial statistics of point patterns. J. Earth Sci. 24, 1-11. 10.1007/s12583-013-0305-6 - DOI
-
- Arroyo, J., Escobar-Zarate, D., Wells, H. H., Constans, M. M., Thao, K., Smith, J. M., Sieben, C. J., Martell, M. R., Kline, T. L., Irazabal, M. V.et al. (2021). The genetic background significantly impacts the severity of kidney cystic disease in the Pkd1RC/RC mouse model of autosomal dominant polycystic kidney disease. Kidney Int. 99, 1392-1407. 10.1016/j.kint.2021.01.028 - DOI - PMC - PubMed
-
- Bell, S. E., Sanchez, M. J., Spasic-Boskovic, O., Santalucia, T., Gambardella, L., Burton, G. J., Murphy, J. J., Norton, J. D., Clark, A. R. and Turner, M. (2006). The RNA binding protein Zfp36l1 is required for normal vascularisation and post-transcriptionally regulates VEGF expression. Dev. Dyn. 235, 3144-3155. 10.1002/dvdy.20949 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- PKD-22-01/Polycystic Kidney Disease Charity
- CTG-DMM23091209/Disease Models and Mechanisms Conference Travel Grant
- CTG-DMM23091209/Disease Models & Mechanisms
- University College London
- MR/P018629/1/MRC_/Medical Research Council/United Kingdom
- PhD2020\100012/Rosetrees Trust
- WT_/Wellcome Trust/United Kingdom
- NIHR Great Ormond Street Hospital Biomedical Research Centre
- 23130027/Central Laser Facility, Science and Technology Facilities Council
- 220895/Z/20/Z)/WT_/Wellcome Trust/United Kingdom
- Specialised Foundation Programme in the East of England Foundation Schools
- 314710/Z/24/Z/WT_/Wellcome Trust/United Kingdom
- Foulkes Foundation
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

