Enhanced T-cell immunity and lower humoral responses following 5-dose SARS-CoV-2 vaccination in patients with inborn errors of immunity compared with healthy controls
- PMID: 40114918
- PMCID: PMC11922935
- DOI: 10.3389/fimmu.2025.1538453
Enhanced T-cell immunity and lower humoral responses following 5-dose SARS-CoV-2 vaccination in patients with inborn errors of immunity compared with healthy controls
Abstract
Objective: Patients with Inborn Errors of Immunity (IEI) are at higher risk of severe SARS-CoV-2 infection. We evaluated humoral and cellular responses to COVID-19 vaccines in Brazilian patients with IEI and healthy controls.
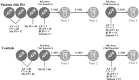
Methods: Fifty-five patients with IEI (13-61 years) and 60 controls (13-71 years) received inactivated SARS-CoV-2 (CoronaVac), non-replicating virus-vectored (ChAdOx1 nCoV-19, AstraZeneca) or monovalent mRNA (Original strain of BNT162b2, Pfizer-BioNTech) and bivalent mRNA (Original/Omicron BA.1, Pfizer-BioNTech) vaccines and were sampled five times. Diagnoses included common variable immunodeficiency (n=25), specific antibody deficiency (n=9), ataxia-telangiectasia (n=5), X-linked agammaglobulinemia (n=4), PIK3CD-related disorders (n=4), hyper-IgM syndrome (n=4), combined immunodeficiency (n=3), and STAT1 gain-of-function (n=1). Humoral immunity was assessed via multiplex microarray for Spike, Nucleocapsid, RBD-Wuhan, RBD-Delta, RBD-BA.1, RBD-BA.2 and RBD-BA.5 neutralizing antibodies. T-cell responses to Spike and Nucleocapsid were assessed using ELISpot.
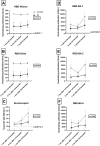
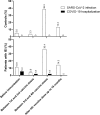
Results: Patients with IEI exhibited significantly lower levels of Nucleocapsid and RBD-neutralizing antibodies (p < 0.05). Notable differences in RBD-BA.2 (p = 0.008) and IgG-Nucleocapsid (p = 0.010) levels emerged over time. T-cell responses to Spike were stronger in patients with IEI post-booster (405 vs. 149 spot-forming cells/million PBMC; p = 0.002). Both groups showed enhanced Nucleocapsid-specific cellular responses over time (p = 0.017). COVID-19 hospitalization rates among patients with IEI with SARS-CoV-2 diagnosis dropped from 33.3% to zero after the first booster dose.
Conclusions: While humoral responses to SARS-CoV-2 vaccines were weaker in patients with IEI, their cellular immunity was similar to controls. Boosters enhanced both humoral and cellular responses. After completion of the vaccination protocol, none of the patients with IEI were hospitalized with COVID-19. Robust T-cell responses may play a critical role in protecting patients with IEI from severe COVID-19 and mortality.
Keywords: COVID-19 vaccines; ELISpot enzyme-linked immunospot; SARS-CoV-2; booster; immune response; inborn errors of immunity; microarray; primary immunodeficiency disorders.
Copyright © 2025 Lopes da Silva, Schmitz, Sullivan, Barbate, de Haro Azinar, Aranda and de Moraes-Pinto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- WHO . COVID-19 dashboard (2024). Available online at: https://data.who.int/dashboards/covid19 (Accessed February 04, 2025).
-
- Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human Inborn Errors of Immunity: 2022 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. (2022) 42:1473–507. doi: 10.1007/s10875-022-01289-3 - DOI - PMC - PubMed
-
- Göschl L, Mrak D, Grabmeier-Pfistershammer K, Stiasny K, Haslacher H, Schneider L, et al. Reactogenicity and immunogenicity of the second COVID-19 vaccination in patients with inborn errors of immunity or mannan-binding lectin deficiency. Front Immunol. (2022) 13:974987. doi: 10.3389/fimmu.2022.974987 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

