Unravelling the complexity of EGFR-mutated lung adenocarcinoma: a unique case report with histological transformations and co-alteration acquisition
- PMID: 40114956
- PMCID: PMC11921265
- DOI: 10.21037/tlcr-24-707
Unravelling the complexity of EGFR-mutated lung adenocarcinoma: a unique case report with histological transformations and co-alteration acquisition
Abstract
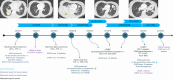
Background: Osimertinib, a third-generation tyrosine kinase inhibitor that targets epidermal growth factor receptor (EGFR), specifically inhibits both EGFR tyrosine kinase inhibitor-sensitive mutations and T790M resistance mutations. Despite initial positive responses to EGFR tyrosine kinase inhibitors, nearly all patients eventually experience disease progression. Mechanisms of resistance are classically divided into EGFR-dependent and EGFR-independent mechanisms, such as the activation of alternative pathways and histological changes. We report a case of histological transformation into large cell carcinoma associated with the subsequent acquisition of an anaplastic lymphoma kinase (ALK) rearrangement after osimertinib exposure.
Case description: A 67-year-old female with no smoking history presented with supraclavicular lymphadenopathy and asthenia, which led to a diagnosis of stage IVB lung adenocarcinoma. Next generation sequencing (NGS) identified an EGFR Ex19del mutation, which suggested the use of afatinib, as it was prescribed prior to osimertinib and was covered by insurance. Initial treatment with afatinib resulted in partial remission, followed by pulmonary progression without the EGFR-T790M mutation. Moreover, ALK and ROS1 were identified through immunohistochemistry (IHC), with ROS1 expression subsequently confirmed by fluorescence in situ hybridization (FISH); this prompted a switch to crizotinib, which was discontinued owing to further disease progression. Osimertinib was then administered, which resulted in a significant positive response; however, after six months pulmonary progression was observed. A subsequent biopsy indicated a transformation to large cell neuroendocrine carcinoma, which led to treatment with platinum-etoposide chemotherapy and, later, paclitaxel and osimertinib, both of which are partially effective. Finally, a new biopsy confirmed ALK positivity in a large cell neuroendocrine carcinoma that was still harbouring an EGFR exon 19 deletion, so alectinib was introduced.
Conclusions: To our knowledge, this case is the first reported incidence of transformation into large cell carcinoma coupled with a second acquisition of alterations in ALK. These findings underscore the necessity of monitoring patients with oncogenic addiction through both liquid biopsy for on-target mechanism detection and tissue sampling to detect histological transformations. These mechanisms can occasionally be combined, thereby providing comprehensive panels at each stage of tumour progression.
Keywords: Epidermal growth factor receptor (EGFR); anaplastic lymphoma kinase (ALK); case report; large-cell neuroendocrine carcinoma (LCNEC); transformation.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-707/coif). J.B.O. reports payment or honoraria for scientific presentations from Astra-Zeneca and Pierre Fabre; payment for expert testimony from Novartis and support for attending meetings and travel from Astra-Zeneca, all outside of the submitted work. G.D. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Chiesi, Sanofi, and GSK (personal fees), outside of the submitted work. J.A. reports grants from AMGEN, and French Innovative Research Fund; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Roche, Pfizer, MSD, Bristol-Myers Squibb, Novartis, AstraZeneca, Takeda, Sanofi, and Amgen; support for attending meetings and/or travel from Roche, Pfizer, MSD, Takeda, and Sanofi. The other authors have no conflicts of interest to declare.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
